Tibrolin D Tablets
Therapy Area
Pain management
1.0 Generic name
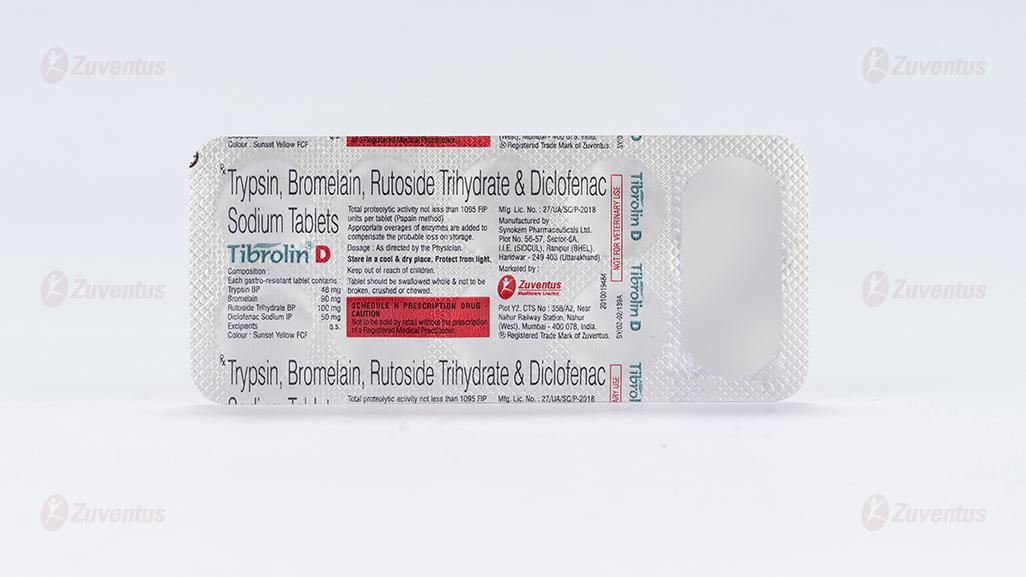
Trypsin, Bromelain, Rutoside Trihydrate & Diclofenac Sodium Tablets
2.0 Qualitative and quantitative composition
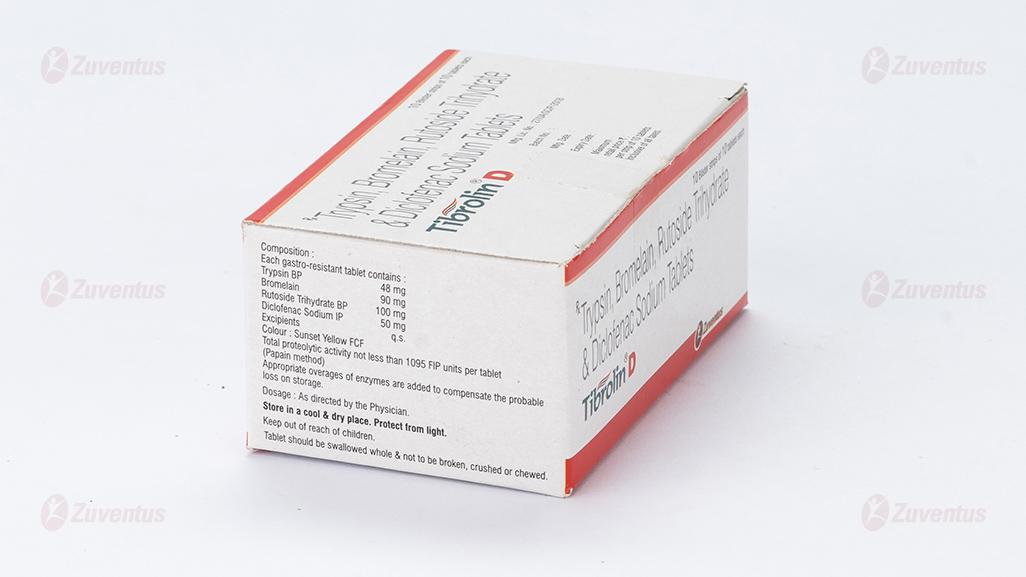
Each gastro-resistant tablet contains :
Trypsin BP 48 mg
Bromelain 90 mg
Rutoside Trihydrate BP 100 mg
Diclofenac Sodium IP 50 mg
Excipients q.s.
Colour : Sunset Yellow FCF
Total proteolytic activity not less than 1095 FIP units per tablet (Papain method)
Appropriate overages of enzymes are added to compensate the probable loss on storage.
3.0 Dosage form and strength
Tablet
Trypsin 48 mg, Bromelain 90 mg, Rutoside Trihydrate 100 mg, Diclofenac Sodium 50 mg.
4.0 Clinical particulars
4.1 Therapeutic indication
- For post-surgical management of wound to reduce the pain and inflammation.
- Treatment of edema and inflammation of traumatic origin, such as contusions, lacerations and cuts.
- Treatment of edema and inflammation following surgery, tooth extractions, cellulitis, abscess, sports injuries and sprains.
- Treatment of inflammatory episodes of rheumatic and degenerative conditions, such as rheumatoid arthritis, osteoarthritis, spondylopathies, tendonitis and bursitis.
4.2 Posology and method of administration
Adults
One tablet 2 - 3 times daily in adults.
Tablets should be swallowed on empty stomach at least 30 min before meals or 2 h after meals.
Children
Tibrolin D is not recommended in children.
4.3 Contraindications
• Hypersensitivity to the active substances or any of the excipients.
• Hypersensitivity to pineapples or papain.
• In the setting of coronary artery bypass graft (CABG) surgery.
• Patients with hereditary coagulation disorders (e.g. hemophilia), severe liver damage and dialyzed patients.
• Active gastric or intestinal ulcer, bleeding or perforation. History of bleeding or perforation, related to previous NSAID therapy.
• Last trimester of pregnancy and breast feeding.
• Hepatic or renal failure.
• Like other NSAIDs, Diclofenac is also contraindicated in patients in whom attacks of asthma, angioedema, urticaria or acute rhinitis are precipitated by ibuprofen, acetylsalicylic acid or other NSAID.
• Established congestive heart failure (NYHA II-IV), ischemic heart disease, peripheral arterial disease and/or cerebrovascular disease.
4.4 Special warnings and precautions for use
Use in patients with cardiopulmonary and pulmonary disease
Tibrolin D should be used with caution in patients with cardiopulmonary and pulmonary disease, including pulmonary burn trauma and suspected pulmonary burn trauma.
Cardiovascular thrombotic events
Non-steroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use. Tibrolin D is contraindicated in the setting of coronary artery bypass graft (CABG) surgery. Patients should remain alert for the signs and symptoms of serious arteriothrombotic events (e.g. chest pain, shortness of breath, weakness, slurring of speech), which can occur without warnings. Patients should be instructed to see a physician immediately in case of such an event.
Patients with congestive heart failure
Patients with congestive heart failure (NYHA-I) or patients with significant risk factors for cardiovascular events (e.g. hypertension, hyperlipidaemia, diabetes mellitus, smoking) should be treated with Tibrolin D only after careful consideration. As the cardiovascular risks of Diclofenac may increase with dose and duration of exposure, the shortest duration possible and the lowest effective daily dose should be used. The patient's need for symptomatic relief and response to therapy should be re-evaluated periodically. Appropriate monitoring and advice are required for patients with a history of hypertension and congestive heart failure (NYHA-I) as fluid retention and oedema have been reported in association with NSAID therapy, including Diclofenac.
Gastrointestinal bleeding, ulceration and perforation
NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk of serious GI events. If GI bleeding or ulceration occurs in patients receiving Tibrolin D, the drug should be withdrawn.
As with all NSAIDs, including Diclofenac, close medical surveillance is imperative and particular caution should be exercised when prescribing Tibrolin D in patients with symptoms indicative of gastrointestinal disorders, or with a history suggestive of gastric or intestinal ulceration, bleeding or perforation.
To reduce the risk of GI toxicity in patients with a history of ulcer, particularly if complicated with haemorrhage or perforation, and in the elderly, the treatment should be initiated and maintained at the lowest effective dose. The risk of GI bleeding, ulceration or per foration is higher with increasing NSAID doses including Diclofenac.
NSAIDs, including Diclofenac, may be associated with increased risk of gastro-intestinal anastomotic leak. Close medical surveillance and caution are recommended when using Tibrolin D after gastro-intestinal surgery.
Use of concomitant medications
The concomitant use of Diclofenac with systemic NSAIDs including cyclooxygenase-2 selective inhibitors should be avoided due to the absence of any evidence demonstrating synergistic benefits and the potential for additive undesirable effects.
Combination therapy with protective agents (e.g. Misoprostol or proton pump inhibitors) should be considered for these patients, and also for patients requiring concomitant use of medicinal products containing low dose Acetylsalicylic acid (ASA / Aspirin or medicinal products likely to increase gastrointestinal risk.
Caution is recommended in patients receiving concomitant medications which could increase the risk of ulceration or bleeding, such as systemic corticosteroids, anticoagulants such as Warfarin, selective serotonin-reuptake inhibitors (SSRIs) or anti-platelet agents such as Acetylsalicylic acid.
Hypersensitivity reaction
As with other nonsteroidal anti-inflammatory drugs including Diclofenac, allergic reactions, including anaphylactic / anaphylactoid reactions, can also occur without earlier exposure to the drug. Hypersensitivity reactions can also progress to Kounis syndrome, a serious allergic reaction that can result in myocardial infarction. Presenting symptoms of such reactions can include chest pain occurring in association with an allergic reaction to Diclofenac.
Skin effects
Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs, including Diclofenac. Patients appear to be at the highest risk of these reactions early in the course of therapy : the onset of the reaction occurring in the majority of cases within the first month of treatment. Tibrolin D should be discontinued at the first appearance of skin rash, mucosal lesions or any other signs of hypersensitivity.
Hepatic effects
As with other NSAIDs, including Diclofenac, values of one or more liver enzymes may increase. During prolonged treatment with Tibrolin D, regular monitoring of hepatic function is indicated as a precautionary measure. Close medical surveillance is required when prescribing Tibrolin D to patients with impairment of hepatic function as their condition may be exacerbated.
If abnormal liver function tests persist or worsen, clinical signs or symptoms consistent with liver disease develop or if other manifestations occur (eosinophilia, rash), Tibrolin D should be discontinued. Hepatitis may occur with Diclofenac without prodromal symptoms. Caution is called for when using Tibrolin D in patients with hepatic porphyria, since it may trigger an attack
Renal effects
As fluid retention and oedema have been reported in association with NSAIDs therapy, including Diclofenac, particular caution is called for in patients with impaired cardiac or renal function, history of hypertension, the elderly, patients receiving concomitant treatment with diuretics or medicinal products that can significantly impact renal function, and those patients with substantial extracellular volume depletion from any cause, e.g. before or after major surgery. Monitoring of renal function is recommended as a precautionary measure when using Tibrolin D in such cases. Discontinuation therapy is usually followed by recovery to the pre-treatment state.
SLE and mixed connective tissue disease
In patients with systemic lupus erythematosus (SLE) and mixed connective tissue disorders there may be an increased risk of aseptic meningitis.
Haematological effects
Use of Diclofenac are recommended only for short term treatment.
During prolonged treatment with Diclofenac, as with other NSAIDs, monitoring of the blood count is recommended. Diclofenac may reversibly inhibit platelet aggregation. Patients with defects of haemostasis, bleeding diathesis or haematological abnormalities should be carefully monitored.
Pre-existing asthma
In patients with asthma, seasonal allergic rhinitis, swelling of the nasal mucosa (i.e. nasal polyps), chronic obstructive pulmonary diseases or chronic infections of the respiratory tract (especially if linked to allergic rhinitis-like symptoms), reactions on NSAIDs like asthma exacerbations (so called intolerance to analgesics / analgesics asthma), Quincke's oedema or urticaria are more frequent than in other patients. Therefore, special precaution is recommended in such patients (readiness for emergency). This is applicable as well for patients who are allergic to other substances, e.g. with skin reactions, pruritus or urticaria.
Like other drugs that inhibit prostaglandin synthetase activity, Diclofenac Sodium and other NSAIDs can precipitate bronchospasm if administered to patients suffering from, or with a previous history of bronchial asthma.
Female fertility
The use of Diclofenac may impair female fertility and is not recommended in women attempting to conceive. In women who may have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of Tibrolin D should be considered.
Other precautions
Tibrolin D does not replace antibiotic treatment of infectious inflammation but increases its effectiveness. Like other NSAIDs, Diclofenac may mask the signs and symptoms of the infection due to its pharmacodynamic properties. Close medical surveillance and caution should be exercised in patients with ulcerative colitis, or with Crohn's disease as these conditions may be exacerbated.
4.5 Drugs interactions
Trypsin, Bromelain and Rutoside Trihydrate
• Bromelain is an inhibitor of cytochrome P 450 2C8 (CYP2C8) and P 450 2C9 (CYP2C9). This should be taken into account if subject FDC is used in patients receiving CYP2C8 substrates (including Amiodarone, Amodiaquine, Chioroquine, Fluvastatin, Paclitaxel, Pioglitazne, Repaglinide, Rosiglitazone, Sorafenib and Torasemide) and CYP2C9 substrates (including Ibuprofen, Tolbutamide, Glipizide, Losartan, Celecoxib, Warfarin and Phenytoin).
• Bromelain may enhance the actions of fluorouracil and vincristine. Patients should be monitored for increased toxicity.
• Bromelain may enhance the hypotensive effect of ACE inhibitors, causing larger decreases in blood pressure than expected. Blood pressure should be monitored in patients receiving ACE inhibitors.
• Bromelain may increase drowsiness caused by some medicinal products (e.g., benzodiazepines, barbiturates, narcotics and antidepressants). This should be taken into account when dosing such products.
Diclofenac
• Lithium : If used concomitantly, Diclofenac may increase plasma concentrations of lithium. Monitoring of the serum Lithium level is recommended.
• Digoxin : If used concomitantly, Diclofenac may raise plasma concentrations of Digoxin. Monitoring of the serum Digoxin level is recommended.
• Diuretics and antihypertensive agents : Like other NSAIDs, concomitant use of Diclofenac with diuretics and antihypertensive agents (e.g. betablockers, angiotensin converting enzyme (ACE) inhibitors may cause a decrease in their antihypertensive effect via inhibition of vasodilatory prostaglandin synthesis.
• Therefore, the combination should be administered with caution and patients, especially the elderly, should have their blood pressure periodically monitored. Patients should be adequately hydrated and consideration should be given to monitoring of renal function after initiation of concomitant therapy periodically thereafter, particularly for diuretics and ACE inhibitors due to the increased risk of nephrotoxicity (see section 4.4 Special warnings and precautions for use).
• Drugs known to cause hyperkalemia : Concomitant treatment with Potassium-sparing diuretics, Ciclosporin, Tacrolimus or Trimethoprim may be associated with increased serum Potassium levels, which should therefore be monitored frequently.
• Anticoagulants and anti-platelet agents : Caution is recommended since concomitant administration could increase the risk of bleeding. Although clinical investigations do not appear to indicate that Diclofenac has an influence on the effect of anticoagulants, there are reports of an increased risk of haemorrhage in patients receiving Diclofenac and anticoagulant concomitantly. Therefore, to be certain that no change in anticoagulant dosage is required, close monitoring of such patients is required. As with other nonsteroidal anti-inflammatory agents, Diclofenac in a high dose can reversibly inhibit platelet aggregation.
• Other NSAIDs including cyclooxygenase-2 selective inhibitors and corticosteroids : Co-administration of Diclofenac with other systemic NSAIDs or corticosteroids may increase the risk of gastrointestinal bleeding or ulceration. Avoid concomitant use of two or more NSAIDs.
• Selective serotonin reuptake inhibitors (SSRIs) : Concomitant administration of SSRI's may increase the risk of gastrointestinal bleeding.
• Antidiabetics : Clinical studies have shown that Diclofenac can be given together with oral antidiabetic agents without influencing their clinical effect. However, there have been isolated reports of hypoglycaemic and hyperglycaemic effects necessitating changes in the dosage of the antidiabetic agents during treatment with Diclofenac. For this reason, monitoring of the blood glucose level is recommended as a precautionary measure during concomitant therapy.
• Methotrexate : Diclofenac can inhibit the tubular renal clearance of Methotrexate hereby increasing methotrexate levels. Caution is recommended when NSAIDs, including Diclofenac, are administered less than 24 hours before treatment with Methotrexate, since blood concentrations of methotrexate may rise and the toxicity of this substance be increase. Cases of serious toxicity have been reported when Methotrexate and NSAIDs, including Diclofenac are given within 24 hours of each other. This interaction is mediated through accumulation of Methotrexate resulting from impairment of renal excretion in the presence of the NSAID.
• Ciclosporin : Diclofenac, like other NSAIDs, may increase the nephrotoxicity of Ciclosporin due to the effect on renal prostaglandins. Therefore, it should be given at doses lower than those that would be used in patients not receiving Ciclosporin.
• Tacrolimus : Possible increased risk of nephrotoxicity when NSAIDs are given with Tacrolimus. This might be mediated through renal antiprostagladin effects of both NSAID and calcineurin inhibitor.
• Quinolone antibacterials : Convulsions may occur due to an interaction between Quinolones and NSAIDs. This may occur in patients with or without a previous history of epilepsy or convulsions. Therefore, caution should be exercised when considering the use of a quinolone in patients who are already receiving an NSAID.
• Phenytoin : When using phenytoin concomitantly with Diclofenac, monitoring of Phenytoin plasma concentrations is recommended due to an expected increase in exposure to Phenytoin.
• Colestipol and Cholestyramine : These agents can induce a delay or decrease in absorption of Diclofenac. Therefore, it is recommended to administer Diclofenac at least one hour before or 4 to 6 hours after administration of Colestipol / Cholestyramine.
• Cardiac glycosides : Concomitant use of cardiac glycosides and NSAIDs in patients may exacerbate cardiac failure, reduce GFR and increase plasma glycoside levels.
• Mifepristone : NSAIDs should not be used for 8 - 12 days after Mifepristone administration as NSAIDs can reduce the effect of Mifepristone.
• Potent CYP2C9 inhibitors : Caution is recommended when co-prescribing Diclofenac with potent CYP2C9 inhibitors (such as Voriconazole), which could result in a significant increase in peak plasma concentrations and exposure to Diclofenac due to inhibition of Diclofenac metabolism.
• Zidovudine : Increased risk of haematological toxicity when NSAIDs are given with Zidovudine. There is evidence of an increased risk of haemarthroses and haematoma in HIV(+) haemophiliacs receiving concurrent treatment with Zidovudine and Ibuprofen.
4.6 Use in special populations
Pregnancy
Inhibition of prostaglandin synthesis may adversely affect the pregnancy and/or the embryo/foetal development. Data from epidemiological studies suggest an increased risk of miscarriage and or cardiac malformation and gastroschisis after use of a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk for cardiovascular malformation was increased from less than 1% up to approximately 1.5%. The risk is believed to increase with dose and duration of therapy. In animals, administration of a prostaglandin synthesis inhibitor has shown to result in increased pre-and post-implantation loss and embryo-foetal lethality.
In addition, increased incidences of various malformations, including cardiovascular, have been reported in animals given a prostaglandin synthesis inhibitor during organogenetic period. If Diclofenac is used by a woman attempting to conceive, or during the 1st trimesters of pregnancy, the dose should be kept as low and duration of treatment as short as possible.
During the third trimester of pregnancy, all prostaglandin synthesis inhibitors may expose the foetus to :
• Cardiopulmonary toxicity. (with premature closure of the ductus arteriosus and pulmonary hypertension.)
• Renal dysfunction, which may progress to renal failure with oligo-hydroamniosis
The mother and the neonate, at the end of the pregnancy, to :
• Possible prolongation of bleeding time, an anti-aggregating effect which may occur even at very low doses.
• Inhibition of uterine contractions resulting in delayed or prolonged labour.
Consequently, Tibrolin D is contra-indicated during the third trimester of pregnancy.
Breast-feeding
Like other NSAIDs, Diclofenac passes into breast milk in small amounts. Therefore, Tibrolin D should not be administered during breast feeding in order to avoid undesirable effects in the infant.
Fertility
Female fertility : As with other NSAIDs, the use of Diclofenac may impair female fertility and is not recommended in women attempting to conceive. In women who may have difficulties conceiving or who are undergoing investigation of infertility, withdrawal of Tibrolin D should be considered.
Paediatric population
There is limited safety data from the use in the paediatric population. Tibrolin D is not indicated for use in patients younger than 18 years.
Geriatric population
Elderly patients, compared to younger patients, are at greater risk for NSAID-associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects. Diclofenac is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Patients should report any unusual abdominal symptoms (especially GI bleeding).
4.7 Effects on ability to drive and use machines
Patients who experience visual disturbances, dizziness, vertigo, somnolence, central nervous system disturbances, drowsiness, or fatigue while taking Tibrolin D should refrain from driving or operating machinery.
4.8 Undesirable effects
Trypsin, Bromelain and Rutoside Trihydrate
Generally, well tolerated, even during prolonged high doses were observed in most cases, side effects. Occasionally may appear insignificant change in consistency, colour and odour of stool. Rare side effects are: headache, hunger, and increased sweating. During high-dose were observed feelings of fullness, impoundment, occasional nausea. This feeling can be avoided by dividing the total dose during the day. Rarely occurring allergic reactions (skin rash) disappear after discontinuation.
Diclofenac
Adverse reactions are ranked under the heading of frequency, the most frequent first, using the following convention : Very common (> 1/10); Common (≥ 1/100, < 1/10); Uncommon (≥ 1/1,000, < 1/100); Rare (≥ 1/10,000, < 1/1000); Very rare (< 1/10,000); Unknown cannot be estimated from available data
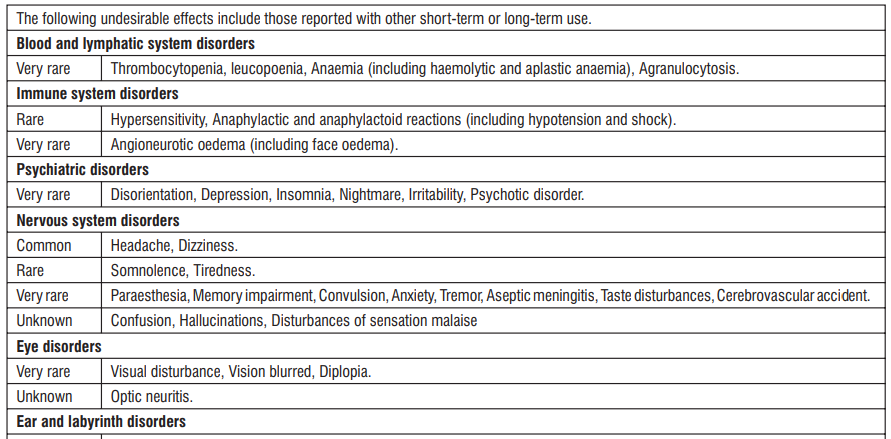
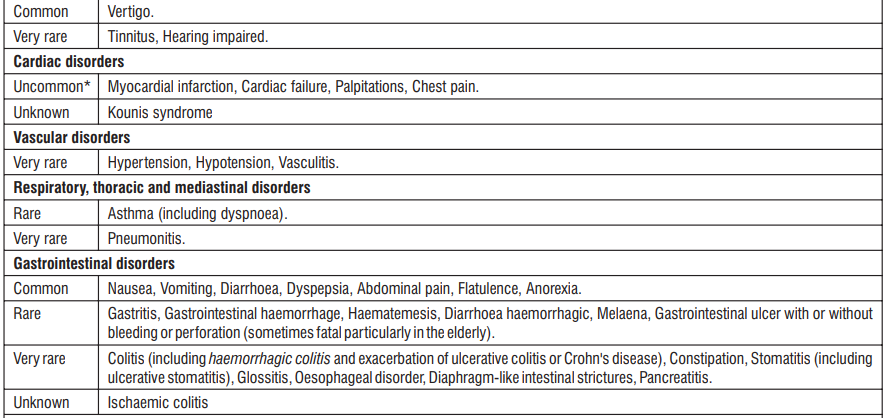
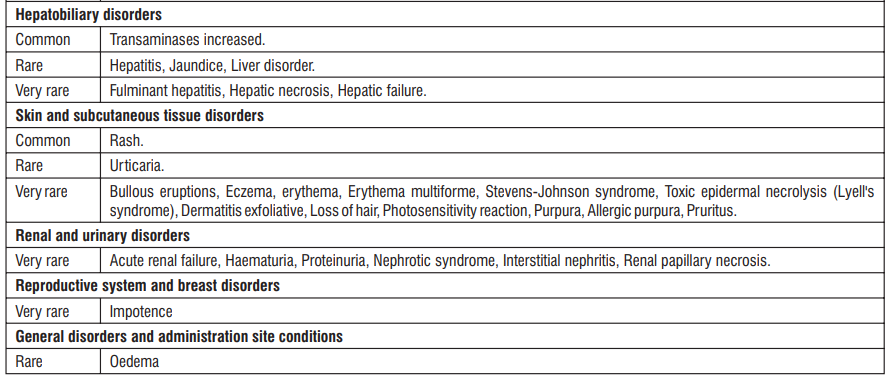
* The frequency reflects data from long-term treatment with a high dose (150 mg/day).
Clinical trial and epidemiological data consistently point towards an increased risk of arterial thrombotic events (for example myocardial infarction or stroke) associated with the use of Diclofenac, particularly at high doses (150 mg daily) and in long term treatment.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com Website : http://www.zuventus.co.in/safety.aspx
4.9 Overdose
Symptoms
There is no typical clinical picture resulting from Diclofenac over dosage. Symptoms include headache, nausea, vomiting, epigastric pain, gastrointestinal bleeding, rarely diarrhoea, dizziness, disorientation, excitation, coma, drowsiness, tinnitus, fainting, occasionally convulsions. In rare cases of significant poisoning acute renal failure and liver damage are possible.
Management of overdoses
Patients should be treated symptomatically as required. Within one hour of ingestion of a potentially toxic amount, activated charcoal should be considered. Alternatively, in adults, gastric lavage should be considered within one hour of ingestion of a potentially life-threatening overdose. Good urine output should be ensured. Special measures such as forced diuresis, dialysis or haemo-perfusion are probably of no help in eliminating NSAIDs, including Diclofenac, due to high protein binding and extensive metabolism. Renal and liver function should be closely monitored. Patients should be observed for at least four hours after ingestion of potentially toxic amounts. Frequent or prolonged convulsions should be treated with intravenous diazepam. Supportive measures should be given for complications such as hypotension, renal failure, gastrointestinal disorder, and respiratory depression. Other measures may be indicated by the patient's clinical condition.
5.0 Pharmacological properties
5.1 Mechanism of action
Trypsin, Bromelain and Rutoside Trihydrate
Trypsin is a proteolytic enzyme found in the digestive system where it hydrolyses proteins. Trypsin breaks the fibrin barrier and restores circulation; resolves edema and hematoma; promotes phagocytosis to remove necrotic debris accumulated during injuries and burns; and inhibits necrotic damage and scarring
Bromelain is a proteolytic enzyme, breaks peptide bonds inside protein molecules. Bromelain assists in removal of blood clot by supporting fibrinolysis, activation of plasmin, preventing aggregation of blood platelets. Bromelain reduces edemas; reduces blood level of plasma kinins to reduce pain; reduces the level of PGE2 and TXA2 in exudates during acute inflammation; induces secretion of interleukin (IL)-1, IL-6, IL-8 and TNF-α to promote re-epithelialization. Bromelain also increases tissue permeability of antibiotics.
Rutoside (also known as Rutin) is the glycoside, formed by combining a disaccharide and flavonol. It is a citrus flavonoid obtained from buckwheat and Pagoda Tree. Rutoside possesses antioxidant, antiinflammatory, antithrombotic, cytoprotective and vasoprotective properties. It promotes wound healing and augments tensile strength of scar tissue significantly. Rutoside also modulates respiratory burst of neutrophils, and inhibits TXA2 formation causing inhibition of platelet aggregation.
Diclofenac
Diclofenac Sodium, a non-steroidal compound, exhibits pronounced antirheumatic, anti-inflammatory, analgesic and antipyretic properties. Its ability to inhibit prostaglandin synthesis is involved in the anti-inflammatory effect.
5.2 Pharmacodynamic properties
Trypsin, Bromelain and Rutoside Trihydrate
The enzymes Bromelain and Trypsin reduce both edema of inflammatory origin, as well as swelling accompanying trauma or surgery. The enzymes show Fibrinolytic and thrombolytic effect, influence on rheological properties of blood, immunomodulatory effect, analgesic effect.
Diclofenac
Diclofenac is a potent inhibitor of prostaglandin biosynthesis and a modulator of arachidonic acid release and uptake. Diclofenac tablets have a rapid onset of action and are therefore suitable for the treatment of acute episodes of pain and inflammation.
5.3 Pharmacokinetic properties
Trypsin, Bromelain and Rutoside Trihydrate
Trypsin
Pharmacokinetic study indicates that Trypsin is absorbed by enterocytes, probably through a transcellular route. Radio-labelled human Trypsin metabolism has been investigated in man. Serum half-life of Trypsin varies from 17.5 - 24 minutes. Around 13 - 38% of the radio-labelled human Trypsin injected was recovered from duodenal juice aspirated continuously over 300 minutes. After Radio-labelled Trypsin infusion, 11% of the total dose was found to be present in the circulation after 75 minutes. Metabolism of Trypsin has not been well documented. Small amount may be excreted in the urine.
Bromelain
Bromelain is absorbed intact through the gastrointestinal tract. It is reported that about 40% of Bromelain is absorbed from the intestine. The highest concentration of Bromelain is found in the blood one hour after administration; however, its proteolytic activity is rapidly deactivated.
Rutoside
Only about 17% of an ingested dose is absorbed. Absorption appears to occur mainly from the colon following the removal of the carbohydrate moiety by bacterial enzymes to form quercetin. Quercetin may undergo glucouronidation in the intestinal cells. Quercetin and glucuronide conjugates of quercetin are transported to the liver via the portal circulation, where they undergo significant first pass metabolism. Gastric secretions can destroy the Trypsin, Rutoside and Bromelain. The high potency standardized Bromelain, Rutoside and Trypsin formulation is protected from stomach acid by a special enteric coating. This allows high levels of these activated proteolytic enzymes to be absorbed by the mucosal membrane of the intestine.
Diclofenac
It is completely absorbed from the enteric-coated tablets after their passage through the stomach. In fasting subjects, the mean peak plasma concentration of 1.5 μg/mL (5 μmol/L) is attained on average 2 hours after ingestion of 50 mg Diclofenac. Diclofenac enters the synovial fluid, and maximum concentrations are measured 2 - 4 hours after the peak plasma. Two hours after reaching the peak, concentrations of the active substance in synovial fluid exceed that of plasma concentrations and remain higher upto 12 hours. Diclofenac becomes bound to serum proteins to the extent of 99.7%, chiefly to albumin (99.4%). The terminal half-life in plasma is 1 to 2 hours. The biotransformation of Diclofenac partly involves glucuronidation of the intact molecule, but mainly single and multiple hydroxylation followed by glucuronidation. About 60% of the administered dose is excreted in the urine in the form of metabolites from one of these two processes. Less than 1% is excreted as unchanged substance. The remainder of the dose is eliminated as metabolites through the bile in the faeces. No pharmacokinetic studies have been conducted to evaluate the pharmacological profile of Tibrolin-D.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Trypsin, Bromelain and Rutoside Trihydrate
Acute median lethal dose after oral administration is too high, cannot be given.
In vivo chromosomal analyses
No statistically significant increase in frequency was observed compared to the control group structural chromosomal aberrations in bone marrow cells.
Embryotoxicity, teratogenicity and impact on pregnancy
There were no documented deviations from normal during pregnancy, there was no increase in the number intra uterine resorption or fetal death was observed and no malformation. There are changes in the development of organs and during ossification. There were no unexpected changes in the representation of gender or weight of the fruit.
Diclofenac
Relevant information on the safety of Diclofenac potassium tablets is included in other sections of the Summary of Product Characteristics.
7.0 Description
Tibrolin D is a fixed dose combination of Trypsin, Bromelain, Rutoside Trihydrate and Diclofenac Sodium.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
PVDC-Alu blister strip of 10 tablets.
8.4 Storage and handing instructions
Store in a cool & dry place. Protect from light.
Keep out of reach of children.
Tablet should be swallowed whole & not to be broken, crushed or chewed.
9.0 Patient counselling information
• Diclofenac in the Tibrolin D
Can cause serious side effects, including: Increased risk of a heart attack or stroke that can lead to death.
This risk may happen early in treatment and may increase : with increasing doses of Diclofenac, with longer use of Diclofenac.
Do not take Diclofenac right before or after a heart surgery called a “coronary artery bypass graft (CABG).”
Avoid taking Diclofenac after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take Diclofenac after a recent heart attack.
Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines: anytime during use, without warning symptoms, that may cause death.
The risk of getting an ulcer or bleeding increases with: past history of stomach ulcers, or stomach or intestinal bleeding with use of Diclofenac, taking medicines called “corticosteroids”, “anticoagulants”, “SSRIs”, or “SNRIs”, increasing doses of Diclofenac, longer use of Diclofenac, smoking, drinking alcohol, older age, poor health, advanced liver disease, bleeding problems
• Tibrolin D should only be used: exactly as prescribed, at the lowest dose possible for your treatment for the shortest time needed.
• Do not take this medicine, if you are allergic to any ingredient of the Tibrolin D, pineapple and papain.
• Signs of an allergic reaction include a rash, itching or shortness of breath.
• Advice patients to talk to your doctor or pharmacist, if you get any side effects. This includes any possible side effects not listed in this leaflet.
• Advice patients If they have any further questions, ask doctor or pharmacist.
Read all of this leaflet carefully before you starts taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, please ask your doctor, pharmacist or nurse.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as you.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
The full name of this medicine is Trypsin, Bromelain, Rutoside Trihydrate and Diclofenac Sodium tablet but within the leaflet it will be referred to as Tibrolin D Tablet.
What is in this leaflet:
1. What Tibrolin D is and what it is used for
2. What you need to know before your child takes Tibrolin D
3. How to take Tibrolin D
4. Possible side effects
5. How to store Tibrolin D
6. Contents of the pack and other Information
What is Tibrolin D and What It is Used for
Tibrolin D is an anti-inflammatory drug. Tibrolin D is used in adults as a supportive treatment for
- Swelling, inflammation or pain as a result of injury
- superficial vein inflammation (thrombophlebitis)
- inflammation of the urinary and genital tract (urogenital tract)
- painful and activated arthroses (chronic joint wear) and
- soft tissue rheumatism (disease with complaints in the area of muscles, tendons, ligaments) dental procedure
Tibrolin D has anti-inflammatory effects. This medicine contains active substances that also reduce swelling and fluid retention (oedema) in the tissues caused by inflammation or injury. The enzymes contained in Tibrolin D also reduce blood clotting by increasing the time it takes for a blood clot to form.
Your doctor will determine how Tibrolin D should be used depending on the symptoms and severity of your disease.
What You Need to Know Before You Take Tibrolin D
Do not take Tibrolin D:
Hypersensitivity to the active substances or any of the excipients.
Hypersensitivity to pineapples or papain.
In the setting of coronary artery bypass graft (CABG) surgery.
Patients with hereditary coagulation disorders (e.g. hemophilia), severe liver damage & dialyzed patients.
Active gastric or intestinal ulcer, bleeding or perforation. History of bleeding or perforation, related to previous NSAID therapy
Last trimester of pregnancy and breast feeding.
Hepatic or renal failure Like other NSAIDs, diclofenac is also contraindicated in patients in whom attacks of asthma, angioedema, urticaria or acute rhinitis are precipitated by ibuprofen, acetylsalicylic acid or other NSAID.
Established congestive heart failure, ischemic heart disease, peripheral arterial disease and/or cerebrovascular disease.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using Tibrolin D
Use in patients with cardiopulmonary and pulmonary disease
Tibrolin D should be used with caution in patients with cardiopulmonary and pulmonary disease, including pulmonary burn trauma and suspected pulmonary burn trauma.
Cardiovascular Thrombotic Events
Tibrolin D is contraindicated in the setting of coronary artery bypass graft (CABG) surgery.
Gastrointestinal Bleeding, Ulceration and Perforation
NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk of serious GI events. Please talk to your doctor or pharmacist before taking Tibrolin D. Before any surgical intervention, the anticoagulant activity of the preparation should be taken into account and the patient should be monitored accordingly. In any case, the preparation should be discontinued 4 days before the operation. As the cardiovascular risks of diclofenac may increase with dose and duration of exposure, the shortest duration possible and the lowest effective daily dose should be used. The patient's need for symptomatic relief and response to therapy should be re-evaluated periodically. Appropriate monitoring and advice are required for patients with a history of hypertension and congestive heart failure as fluid retention and oedema have been reported in association with NSAID therapy, including diclofenac. If GI bleeding or ulceration occurs in patients receiving Tibrolin D, the drug should be withdrawn.
Children and young people
For children and adolescents under the age of 18 there is not enough experience in the mentioned areas of application.
Taking Tibrolin D together with other medicines
Bromelain may enhance the actions of fluorouracil and vincristine. Patients should be monitored for increased toxicity.
Bromelain may enhance the hypotensive effect of ACE inhibitors, causing larger decreases in blood pressure than expected. Blood pressure should be monitored in patients receiving ACE inhibitors.
Bromelain may increase drowsiness caused by some medicinal products (e.g, benzodiazepines, barbiturates, narcotics and antidepressants). This should be taken into account when dosing such products.
Please tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription.
When Tibrolin and antibiotics are administered at the same time, the plasma and urine levels of antibiotics, especially tetracyclines, sulfonamides and amoxicillin, are increased. The dose of antibiotics will be adjusted accordingly by your doctor.
You must not take Tibrolin if you are using other medications that affect blood clotting.
The inhibition of blood clotting can be increased if medicines that affect blood clotting are taken at the same time.
Diclofenac
Lithium: If used concomitantly, diclofenac may increase plasma concentrations of lithium. Monitoring of the serum lithium level is recommended.
Digoxin: If used concomitantly, diclofenac may raise plasma concentrations of digoxin. Monitoring of the serum digoxin level is recommended.
Diuretics and antihypertensive agents: Like other NSAIDs, concomitant use of diclofenac with diuretics and antihypertensive agents (e.g. beta-blockers, angiotensin converting enzyme (ACE) inhibitors may cause a decrease in their antihypertensive effect via inhibition of vasodilatory prostaglandin synthesis.
Therefore, the combination should be administered with caution and patients, especially the elderly, should have their blood pressure periodically monitored. Patients should be adequately hydrated and consideration should be given to monitoring of renal function after initiation of concomitant therapy periodically thereafter, particularly for diuretics and ACE inhibitors due to the increased risk of nephrotoxicity.
Drugs known to cause hyperkalemia: Concomitant treatment with potassium-sparing diuretics, ciclosporin, tacrolimus or trimethoprim may be associated with increased serum potassium levels, which should therefore be monitored frequently.
Anticoagulants and anti-platelet agents: Caution is recommended since concomitant administration could increase the risk of bleeding. Although clinical investigations do not appear to indicate that diclofenac has an influence on the effect of anticoagulants, there are reports of an increased risk of haemorrhage in patients receiving diclofenac and anticoagulant concomitantly. Therefore, to be certain that no change in anticoagulant dosage is required, close monitoring of such patients is required. As with other nonsteroidal anti-inflammatory agents, diclofenac in a high dose can reversibly inhibit platelet aggregation.
Other NSAIDs including cyclooxygenase-2 selective inhibitors and corticosteroids: Co-administration of diclofenac with other systemic NSAIDs or corticosteroids may increase the risk of gastrointestinal bleeding or ulceration. Avoid concomitant use of two or more NSAIDs.
Selective serotonin reuptake inhibitors (SSRIs): Concomitant administration of SSRI's may increase the risk of gastrointestinal bleeding.
Antidiabetics: Clinical studies have shown that diclofenac can be given together with oral antidiabetic agents without influencing their clinical effect. However, there have been isolated reports of hypoglycaemic and hyperglycaemic effects necessitating changes in the dosage of the antidiabetic agents during treatment with diclofenac. For this reason, monitoring of the blood glucose level is recommended as a precautionary measure during concomitant therapy.
Methotrexate: Diclofenac can inhibit the tubular renal clearance of methotrexate hereby increasing methotrexate levels. Caution is recommended when NSAIDs, including diclofenac, are administered less than 24 hours before treatment with methotrexate, since blood concentrations of methotrexate may rise and the toxicity of this substance be increase. Cases of serious toxicity have been reported when methotrexate and NSAIDs, including diclofenac are given within 24 hours of each other. This interaction is mediated through accumulation of methotrexate resulting from impairment of renal excretion in the presence of the NSAID.
Ciclosporin: Diclofenac, like other NSAIDs, may increase the nephrotoxicity of ciclosporin due to the effect on renal prostaglandins. Therefore, it should be given at doses lower than those that would be used in patients not receiving ciclosporin.
Tacrolimus: Possible increased risk of nephrotoxicity when NSAIDs are given with tacrolimus. This might be mediated through renal antiprostagladin effects of both NSAID and calcineurin inhibitor.
Quinolone antibacterials: Convulsions may occur due to an interaction between quinolones and NSAIDs. This may occur in patients with or without a previous history of epilepsy or convulsions. Therefore, caution should be exercised when considering the use of a quinolone in patients who are already receiving an NSAID.
Phenytoin: When using phenytoin concomitantly with diclofenac, monitoring of phenytoin plasma concentrations is recommended due to an expected increase in exposure to phenytoin.
Colestipol and cholestyramine: These agents can induce a delay or decrease in absorption of diclofenac. Therefore, it is recommended to administer diclofenac at least one hour before or 4 to 6 hours after administration of colestipol/ cholestyramine.
Cardiac glycosides: Concomitant use of cardiac glycosides and NSAIDs in patients may exacerbate cardiac failure, reduce GFR and increase plasma glycoside levels.
Mifepristone: NSAIDs should not be used for 8-12 days after mifepristone administration as NSAIDs can reduce the effect of mifepristone.
Potent CYP2C9 inhibitors: Caution is recommended when co-prescribing diclofenac with potent CYP2C9 inhibitors (such as voriconazole), which could result in a significant increase in peak plasma concentrations and exposure to diclofenac due to inhibition of diclofenac metabolism.
Zidovudine: Increased risk of haematological toxicity when NSAIDs are given with zidovudine. There is evidence of an increased risk of haemarthroses and haematoma in HIV(+) haemophiliacs receiving concurrent treatment with zidovudine and ibuprofen.
Use in Special Population:
Pregnancy, lactation and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine, like any other medicine. There are no adequate studies on the use of Tibrolin D during pregnancy and breastfeeding. Tibrolin D must therefore not be used on pregnant or breastfeeding women. Studies on the safety of Tibrolin D do not reveal any dangers with regard to the ability to conceive and/or give birth to humans.
Effects on ability to drive and use machines
Patients who experience visual disturbances, dizziness, vertigo, somnolence, central nervous system disturbances, drowsiness, or fatigue while taking Tibrolin D should refrain from driving or operating machinery.
How to Take Tibrolin D
- Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
- To be taken by mouth
How much to take
1 Tablet 2-3 times daily in adults. Tablets should be swallowed on empty stomach at least 30 min before meals or 2 h after meals.
Children
Tibrolin-D is not recommended in children.
If you take more Tibrolin D than you should
Contact your doctor immediately for advice. Take the medicine pack with you. This is so the doctor knows what you have taken.
If you forget to take Tibrolin D
Try to give Tibrolin D as prescribed. However, if you misses a dose, just resume the usual schedule. Do not give a double dose to make up for a forgotten dose.
Possible Side Effects
Generally, well tolerated, even during prolonged high doses were observed in most cases, side effects. Occasionally may appear insignificant change in consistency, colour and odour of stool. Rare side effects are: headache, hunger, and increased sweating. During high-dose were observed feelings of fullness, impoundment, and occasional nausea. This feeling can be avoided by dividing the total dose during the day. Rarely occurring allergic reactions (skin rash) disappear after discontinuation.
Stop taking Diclofenac Sodium tablets and contact a doctor straight away if you notice:
Uncommon (may affect up to 1 in 100 people):
• Sudden and crushing chest pain (signs of myocardial infarction or heart attack), especially if you have been taking a higher dose (150mg per day) for a long period of time.
• Breathlessness, difficulty breathing when lying down, swelling of the feet or legs (signs of heart failure).
Rare (may affect up to 1 in 1,000 people):
• Any sign of bleeding in the stomach or intestine (e.g. having black tarry stools or blood in your vomit). Mild cramping and tenderness of the abdomen, starting shortly after the start of the treatment with Diclofenac Sodium tablets and followed by bleeding or bloody diarrhoea usually within 24 hours of the onset of abdominal pain. Gastritis (inflammation, irritation or swelling of the stomach lining).
• Stomach ulcers (there have been very rare reported cases resulting in death, particularly in the elderly).
• Allergic reactions which can include skin rash, itching, bruising, painful red areas, peeling or blistering. Facial swelling, serious skin rashes including Stevens-Johnson syndrome and Lyell’s syndrome and other skin rashes which may be made worse by exposure to sunlight. Wheezing or shortness of breath (bronchospasm). Swollen face, lips, hands or fingers.
• Yellowing of your skin or the whites of your eyes.
Very rare (may affect up to 1 in 10,000 people):
• Sudden weakness or numbness in the face, arm or leg especially on one side of the body; sudden loss or disturbance of vision; sudden difficulty in speaking or ability to understand speech; sudden migraine-like headaches which happen for the first time, with or without disturbed vision. These symptoms can be an early sign of a stroke.
• An unexpected change in the amount of urine produced and/or its appearance.
• Inflammation of the lining of the brain (meningitis).
• Fits.
• Persistent sore throat or high temperature. If you notice that you are bruising more easily than usual or have frequent sore throats or infections, tell your doctor. Not known (frequency cannot be estimated from the available data):
• Chest pain, which can be a sign of a potentially serious allergic reaction called Kounis syndrome. Tell your doctor if you experience any of the following: Common (may affect up to 1 in 10 people):
• Stomach pain, heartburn, nausea (feeling sick), vomiting (being sick), diarrhoea, indigestion, wind, loss of appetite.
• Headache, dizziness, vertigo.
• Skin rash or spots.
• Raised levels of liver enzymes in the blood.
Uncommon (may affect up to 1 in 100 people):
• Fast or irregular heartbeat (palpitations), chest pain, heart disorders. Rare (may affect up to 1 in 1,000 people):
• Drowsiness, tiredness.
• Skin rash and itching.
• Fluid retention, symptoms of which include swollen ankles.
• Liver function disorders, including hepatitis and jaundice, inflammation of the pancreas.
• Asthma (symptoms may include wheezing, breathlessness, coughing and a tightness across the chest). Very rare (may affect up to 1 in 10,000 people):
• Tingling or numbness in the fingers, tremor, visual disturbances such as blurred or double vision, taste changes, hearing loss or impairment, tinnitus (ringing in the ears), sleeplessness, nightmares, mood changes, depression, anxiety, irritability, mental disorders, disorientation and loss of memory, headaches together with a dislike of bright lights, fever and a stiff neck.
• Constipation, inflammation of the tongue, mouth ulcers, inflammation of the inside of the mouth or lips, lower gut disorders (including inflammation of the colon or worsening of ulcerative colitis or Crohn’s disease).
• Hypertension (high blood pressure), hypotension (low blood pressure, symptoms of which may include faintness, giddiness or light headedness), inflammation of blood vessels (vasculitis), inflammation of the lung (pneumonitis), blood disorders (including anaemia).
• Kidney or severe liver disorders including liver failure, presence of blood or protein in the urine.
• Hair loss
. • Impotence.
Not known (frequency cannot be estimated from the available data):
• Throat disorders, confusion, hallucinations, malaise (general feeling of discomfort), inflammation of the nerves in the eye, disturbances of sensation. Medicines such as diclofenac may be associated with a small increased risk of heart attack or stroke.
Reporting of side effects: If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
By reporting side effects you can also provide more information on the safety of this medicine.
How to Store Tibrolin D
- Store protected from light & moisture.
- Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
- Keep away from the reach of children.
Contents of the pack and other information
The active substances are Trypsin, Bromelain, Rutoside Trihydrate and Diclofenac Sodium. Each tablet contains 48 mg Trypsin, 90 mg Bromelain, 100 mg Rutoside and 50 mg Diclofenac.
Marketing authorisation holder:
Zuventus Healthcare Ltd. 5119, Oberoi Garden Estates, D-Wing, Chandivali, Andheri (E), Mumbai 400 072.
Manufactured by:
Synokem Pharmaceuticals Ltd., Plot No. 56-57, Sector-6A, I.I.E. (SIDCUL), Ranipur (BHEL), Haridwar - 249 403 (Uttarakhand).