Tibrolin Tablets
Therapy Area
Pain management
1.0 Generic name
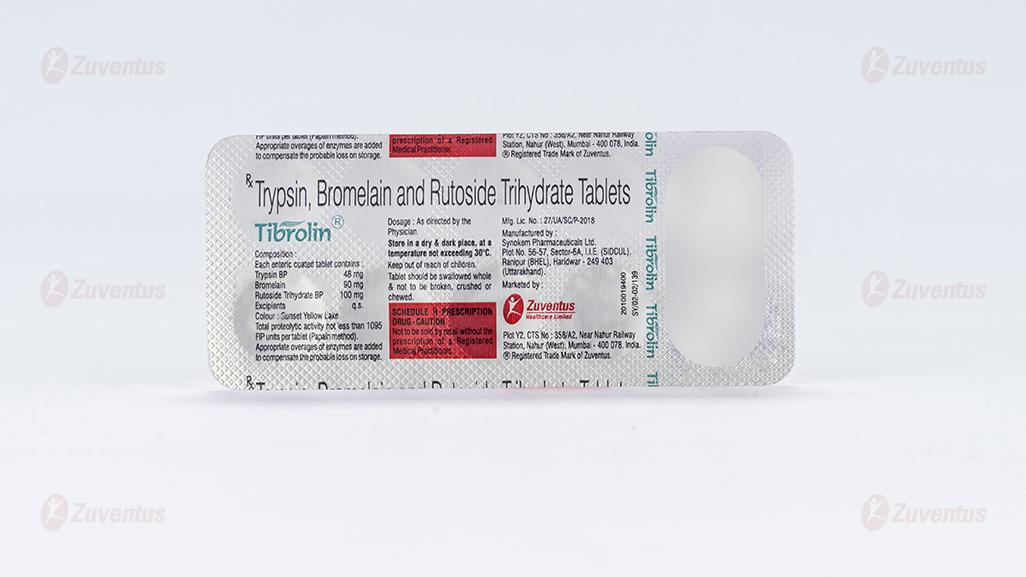
Trypsin, Bromelain and Rutoside Trihydrate Tablets
2.0 Qualitative and quantitative composition
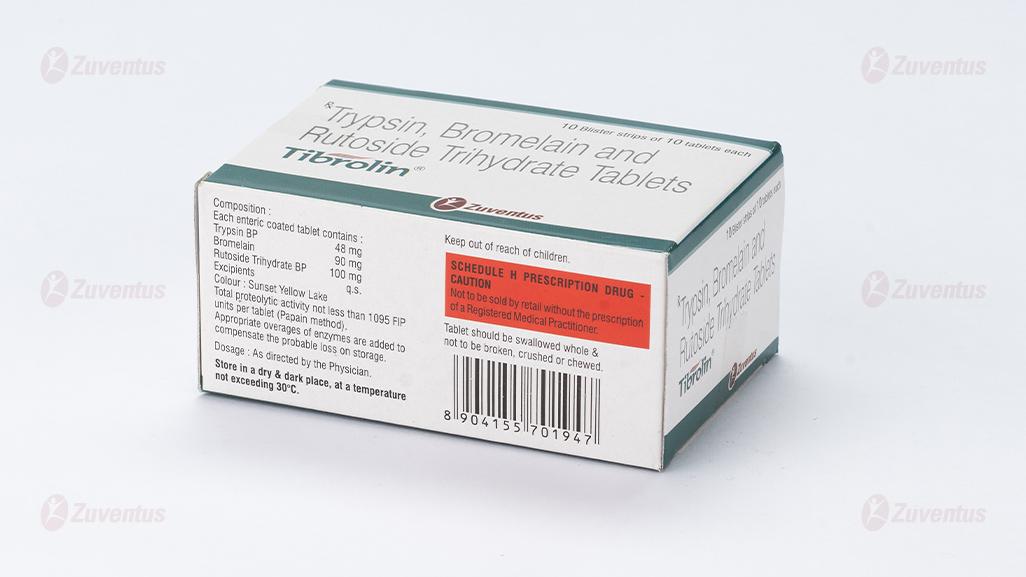
Each enteric coated tablet contains :
Trypsin BP 48 mg
Bromelain 90 mg
Rutoside Trihydrate BP 100 mg
Excipients q.s.
Colour : Sunset Yellow Lake
Total proteolytic activity not less than 1095 FIP units per tablet (Papain method).
Appropriate overages of enzymes are added to compensate the probable loss on storage
3.0 Dosage form and strength
Tablet
4.0 Clinical particulars
4.1 Therapeutic indication
For postoperative inflammation in subject undergoing minor surgery and dental procedure.
4.2 Posology and method of administration
- Adults : 1 to 2 tab b.i.d. or t.i.d. orally
- Children : 1 tab/10 kg body wt, taken daily in 2 - 4 partial doses
Tablets should be swallowed on empty stomach at least 30 min before meals or 2 h after meals.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in the formulation.
4.4 Special warnings and precautions for use
- Should not be used in known cases of hypersensitivity to any of its ingredients.
- Not recommended in patients with hereditary coagulation disorder (e.g. hemophilia) & liver disorder.
- The combination cannot replace an ongoing antibiotic therapy.
- Temporary reduction of dose advised if there is exacerbation of symptoms on initial therapy.
4.5 Drugs interactions
No evidence of incompatibility of Tibrolin with other concomitant medications has been reported.
4.6 Use in special populations
Pregnancy & Breastfeeding
Should be used with caution during pregnancy & breast feeding
4.7 Effects on ability to drive and use machines
It is not known whether Tibrolin tablet alters the ability to drive. Do not drive if you experience any symptoms that affect your ability to concentrate and react.
4.8 Undesirable effects
Generally, well tolerated, even during prolonged high doses were observed in most cases, side effects. Occasionally may appear insignificant change in consistency, colour and odour of stool. Rare side effects are: headache, hunger, and increased sweating. During high-dose were observed feelings of fullness, impoundment, occasional nausea. This feeling can be avoided by dividing the total dose during the day. Rarely occurring allergic reactions (skin rash) disappear after discontinuation
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
Website : https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Even if long-term use of higher doses, there were no toxic effects, including possible light diarrhea that after discontinuing the plant disappears spontaneously.
5.0 Pharmacological properties
5.1 Mechanism of action
Trypsin is a proteolytic enzyme found in the digestive system where it hydrolyses proteins. Trypsin breaks the fibrin barrier and restores circulation; resolves edema and hematoma; promotes phagocytosis to remove necrotic debris accumulated during injuries and burns; and inhibits necrotic damage and scarring.
Bromelain is a proteolytic enzyme, breaks peptide bonds inside protein molecules. Bromelain assists in removal of blood clot by supporting fibrinolysis, activation of plasmin, preventing aggregation of blood platelets. Bromelain reduces edemas; reduces blood level of plasma kinins to reduce pain; reduces the level of PGE2 and TXA2 in exudates during acute inflammation; induces secretion of interleukin (IL)-1, IL-6, IL-8 & TNF-α to promote re-epithelialization. Bromelain also increases tissue permeability of antibiotics.
Rutoside (also known as Rutin) is the glycoside, formed by combining a disaccharide and flavonol. It is a citrus flavonoid obtained from buckwheat & Pagoda Tree. Rutoside possesses antioxidant, antiinflammatory, antithrombotic, cytoprotective & vasoprotective properties. It promotes wound healing & augments tensile strength of scar tissue significantly. Rutoside also modulates respiratory burst of neutrophils, and inhibits TXA2 formation causing inhibition of platelet aggregation
5.2 Pharmacodynamic properties
The enzymes bromelain and trypsin reduce both edema of inflammatory origin, as well as swelling accompanying trauma or surgery. The enzymes show Fibrinolytic and thrombolytic effect, influence on rheological properties of blood, immunomodulatory effect, analgesic effect.
5.3 Pharmacokinetic properties
Trypsin : Pharmacokinetic study indicates that Trypsin is absorbed by enterocytes, probably through a transcellular route. Radio-labelled human Trypsin metabolism has been investigated in man. Serum half life of Trypsin varies from 17.5-24 minutes. Around 13-38% of the radio-labelled human trypsin injected was recovered from duodenal juice aspirated continuously over 300 minutes. After Radio-labelled Trypsin infusion, 11% of the total dose was found to be present in the circulation after 75 minutes. Metabolism of trypsin has not been well documented. Small amount may be excreted in the urine.
Bromelain : Bromelain is absorbed intact through the gastrointestinal tract. It is reported that about 40% of Bromelain is absorbed from the intestine. The highest concentration of bromelain is found in the blood one hour after administration; however, its proteolytic activity is rapidly deactivated.
Rutoside : Only about 17% of an ingested dose is absorbed. Absorption appears to occur mainly from the colon following the removal of the carbohydrate moiety by bacterial enzymes to form quercetin. Quercetin may undergo glucouronidation in the intestinal cells. Quercetin and glucuronide conjugates of quercetin are transported to the liver via the portal circulation, where they undergo significant first pass metabolism.
Gastric secretions can destroy the Trypsin, Rutoside and Bromelain. The high potency standardized Bromelain, Rutoside and Trypsin formulation is protected from stomach acid by a special enteric coating. This allows high levels of these activated proteolytic enzymes to be absorbed by the mucosal membrane of the intestine.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Acute median lethal dose after oral administration is too high, cannot be given. In vivo chromosomal analyzes :
No statistically significant increase in frequency was observed compared to the control group structural chromosomal aberrations in bone marrow cells.
Embryotoxicity, teratogenicity and impact on pregnancy :
There were no documented deviations from normal during pregnancy, there was no increase in the number intra uterine resorption or fetal death was observed and no malformation. There are changes in the development of organs and during ossification. There were no unexpected changes in the representation of gender or weight of the fruit.
7.0 Description
Tibrolin is a combination of two proteases - Trypsin & Bromelain, and a bioflavonoid Rutoside.
Trypsin is a serine protease found in the digestive system, where it cleaves peptide chains, hydrolyses proteins. Trypsin is produced in the pancreas as the inactive proenzyme trypsinogen. Trypsin is obtained from the pancreas of pigs by repeated refining and subsequent activation of the proenzyme trypsinogen.
Bromelain is obtained from crude, aqueous extract from the stems and immature fruits of pineapples (Ananas comosus Merr.). Bromelain constitutes an unusually complex mixture of different thiol-endopeptidases and other components such as phosphatases, glucosidases, peroxidases, cellulases, glycoproteins and carbohydrates, among others. In addition, bromelain contains several proteinase inhibitors.
Rutin, also called rutoside, quercetin-3-rutinoside and sophorin, is a citrus flavonoid glycoside found in buckwheat, the leaves and petioles of Rheum species, and asparagus. Rutin is the glycoside between the flavonols quercetin and the disaccharide rutinose.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None
8.2 Shelf-life
24 Months.
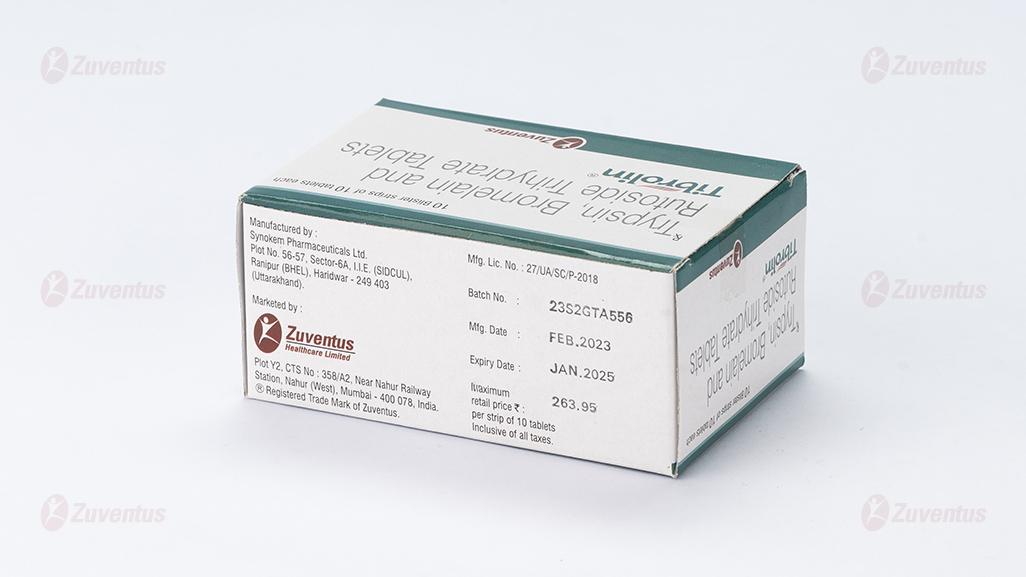
8.3 Packaging information
A blister strip of 10 tablets.
8.4 Storage and handing instructions
- Store in a dry & dark place, at a temperature not exceeding 30°C.
- Keep out of reach of children.
- Tablet should be swallowed whole & not to be broken, crushed or chewed.
9.0 Patient counselling information
- Tibrolin should only be used : exactly as prescribed, at the lowest dose possible for your treatment for the shortest time needed,
- Do not take this medicine, if you are allergic to any ingredient of the Tibrolin
- Signs of an allergic reaction include a rash, itching or shortness of breath.
- Advice patients to talk to your doctor or pharmacist, if you get any side effects. This includes any possible side effects not listed in this leaflet.
- Advice patients If they have any further questions, ask doctor or pharmacist.
12.0 Date of revision
28 April 2021
About Leaflet
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What Tibrolin® Tablets is and what it is used for
- What you need to know before you are given Tibrolin®
- How to use Tibrolin® 4. Possible side effects
- How to store Tibrolin®
- Contents of the pack and other information
1. What is Tibrolin® and what it is used for
Tibrolin® is a combination enzyme supplements which contains a mix of enzymes such as bromelain (from pineapple), trypsin, and rutoside (a bioflavonoid).
- Bromelain: Bromelain is a mixture of proteolytic enzymes derived from the pineapple plant. It is believed to have anti-inflammatory properties by reducing the production of pro-inflammatory cytokines and promoting the breakdown of proteins that contribute to swelling and inflammation.
- Trypsin: Trypsin is a protein-digesting enzyme naturally produced by the pancreas. It aids in the digestion of proteins and has been used in supplements to support the body's natural inflammatory response.
- Rutoside: Rutoside, also known as rutin, is a flavonoid found in certain fruits and vegetables. It is an antioxidant and is thought to help strengthen capillaries and reduce inflammation.
Tibrolin® is commonly used for the following purposes:
- Joint Health: It is often taken to support joint health and mobility, particularly in conditions involving inflammation such as osteoarthritis.
- Inflammatory Conditions: Due to its anti-inflammatory properties, Tibrolin® may be used to help reduce swelling and inflammation in various parts of the body especially post-operatively.
- Injuries: Some athletes and active individuals use Tibrolin® to aid in recovery from injuries or to alleviate muscle soreness after intense exercise.
The enzymes bromelain and trypsin reduce both edema of inflammatory origin, as well as swelling accompanying trauma or surgery.
2. What you need to know before you are given Tibrolin®
Do not use Tibrolin® if: you are allergic to Tibrolin® or any of the other ingredients of this medicine.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Tibrolin® if:
- you have hereditary coagulation disorder (e.g. haemophilia)
- you have liver problems
- The combination cannot replace an ongoing antibiotic therapy.
- Temporary reduction of dose advised if there is exacerbation of symptoms on initial therapy.
Other medicines and Tibrolin®
Tell your doctor, pharmacist or nurse if you are using, have recently used or might use any other medicines.
Currently there are no reports of incompatibility of Tibrolin with other concomitant medications has been reported.
Pregnancy, breast-feeding and fertility
If you are pregnant, think that you might be pregnant or are planning to have a baby, ask your doctor, pharmacist or nurse for advice before being given this medicine. They will decide whether or not you are given this medicine while you are pregnant. Tell your doctor if you are breast-feeding, before being given this medicine. He/she will decide whether or not you can keep breast-feeding, while you are given F Tibrolin®. Tibrolin® should be used with caution during pregnancy & breast feeding.
Driving and using machines
It is not known whether Tibrolin tablet alters the ability to drive. Do not drive if you experience any symptoms that affect your ability to concentrate and react.
3. How to use Tibrolin®
Adults: 1 to 2 tablets two or three times daily by oral route. Tablets should be swallowed on empty stomach at least 30 min before meals or 2 h after meals. Tablet should be swallowed whole & not to be broken, crushed or chewed.
If you have more Tibrolin® than you should
If you think you have been given too much Tibrolin® or if you are agitated, talk to your doctor or nurse straight away.
If you forget to have Tibrolin® Take the forgotten tablet as soon as you remember. Take the next tablet 24 hours after this and continue taking your tablets every 24 hours. Do not take a double dose to make up for a forgotten tablet.
If you stop having Tibrolin® Do not stop having this medicine without first talking to your doctor, pharmacist or nurse. If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Generally, well tolerated, even during prolonged high doses were observed in most cases, side effects.
Occasionally may appear insignificant change in consistency, colour and odour of stool. Rare side effects are: headache, hunger, and increased sweating.
During high-dose were observed feelings of fullness, impoundment, occasional nausea. This feeling can be avoided by dividing the total dose during the day.
Rarely occurring allergic reactions (skin rash) disappear after discontinuation.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top end of the home page.
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store Tibrolin®
Store in a dry & dark place, at a temperature not exceeding 30°C.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label of the vial.
The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions.
6. Contents of the pack and other information
What Tibrolin® contains:
Each enteric coated tablet contains: The active substances are Trypsin (48mg), Bromelain (90mg) and Rutoside Trihydrate (100mg).
What Tibrolin® looks like and contents of the pack
Pack size: A blister strip of 10 tablets.
Marketing Authorisation Holder
Zuventus Healthcare Ltd
Oberoi Garden Estates, D-Wing,
Chandivali, Andheri (E),
Mumbai 400 072. TM - Trade Mark Owners.
This leaflet was last revised in 01/07/2024.