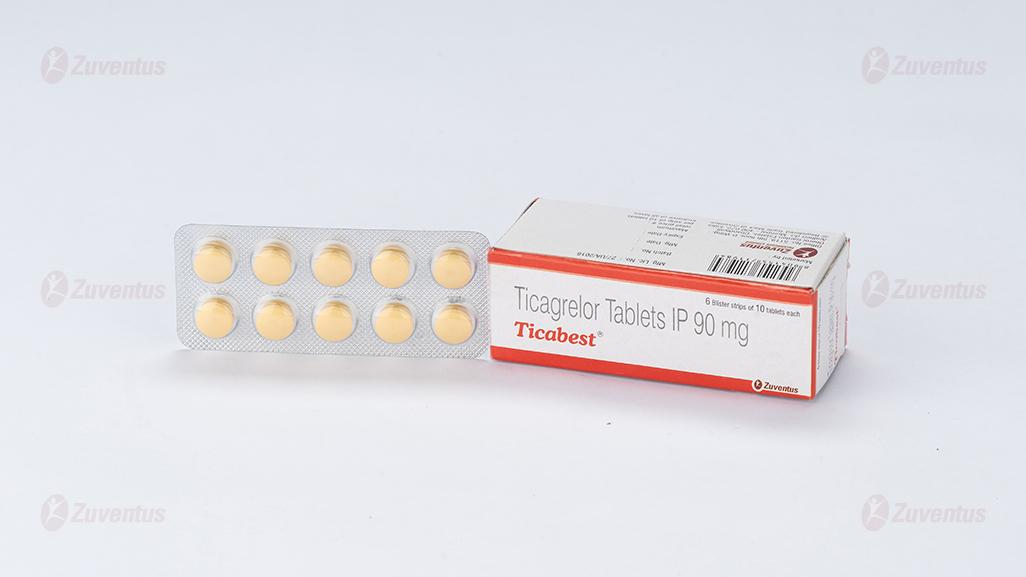
Ticabest Tablets
Therapy Area
Cardiology
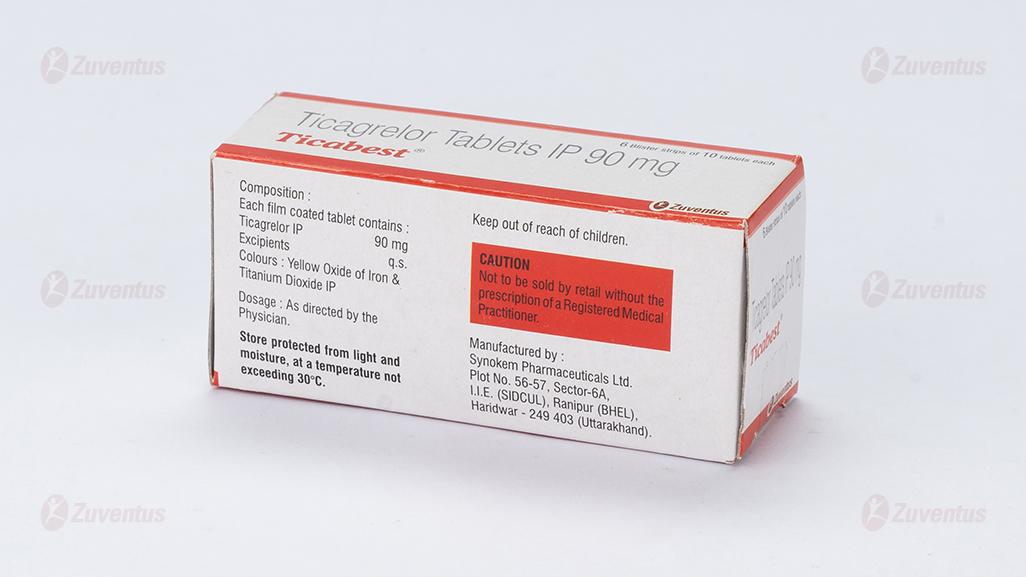
Composition
Each film coated tablet contains :
Ticagrelor IP 90 mg
Excipients q.s
Colours : Yellow Oxide of Iron and Titanium Dioxide IP
Warning : (a) Bleeding risk, and (b) Aspirin dose and Ticagrelor effectiveness
(a) BLEEDING RISK
• Ticagrelor, like other antiplatelet agents, can cause significant, sometimes fatal bleeding.
• Do not start Ticagrelor in patients undergoing urgent coronary artery bypass graft surgery (CABG)
• If possible, manage bleeding without discontinuing Ticagrelor. Stopping Ticagrelor increases the risk of subsequent cardiovascular events.
(b) ASPIRIN DOSE AND TICAGRELOR EFFECTIVENESS
• Maintenance doses of aspirin above 100 mg reduce the effectiveness of Ticagrelor and should be avoided.
Description
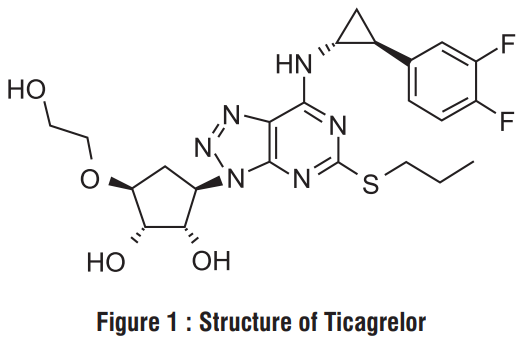
Ticabest contains Ticagrelor, a cyclopentyltriazolopyrimidine, inhibitor of platelet activation and aggregation mediated by the P2Y12 ADP -receptor. Chemically it is (1S,2S,3R,5S)-3-[7-[[(1R,2S)-2-(3,4-difluorophenyl) cyclopropyl]amino] -5-propylsulfanyltriazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol. The empirical formula of Ticagrelor is C23H28F2N6O4S and its molecular weight is 522.57 g/mol. The chemical structure of Ticagrelor is :
Dosage forms and strengths
Ticagrelor is available as a film coated tablets 90 mg for twice daily oral administration.
Indications
Ticagrelor is indicated for the prevention of thrombotic events (cardiovascular death, myocardial infarction and stroke) in patients with Acute coronary syndromes (ACS) unstable angina, non ST elevation Myocardial infarction (STEMI) including patients managed medically, and those who are managed with percutaneous coronary intervention (PCI) or coronary artery by-pass grafting (CABG).
Dose and method of administration
Dosing
In the management of ACS, initiate treatment with 180 mg oral loading dose following an ACS event. Continue treatment with 90 mg twice daily during the first year after an ACS event. Do not administer Ticagrelor with another oral P2Y12 platelet inhibitor. Use Ticagrelor with a daily maintenance dose of aspirin of 75 - 100 mg. A patient who misses a dose of Ticagrelor should take one tablet (their next dose) at its scheduled time.
Administration
For patients who are unable to swallow tablets whole, Ticagrelor tablets can be crushed, mixed with water and drunk. The mixture can also be administered via a nasogastric tube (CH8 or greater).
Use in specific populations
Pregnancy
Pregnancy category : C
There are no adequate and well-controlled studies of Ticagrelor use in pregnant women. In animal studies, Ticagrelor caused structural abnormalities at maternal doses about 5 to 7 times the maximum recommended human dose (MRHD) based on body surface area. Ticagrelor should be used during pregnancy only if the potential benefit justifes the potential risk to the fetus.
Nursing mothers
It is not known whether Ticagrelor or its active metabolites are excreted in human milk. Ticagrelor is excreted in rat milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from Ticagrelor, a decision should be made whether to discontinue nursing or to discontinue Ticagrelor.
Pediatric use
The safety and effectiveness of Ticagrelor in pediatric patients have not been established.
Geriatric use
No differences in safety or effectiveness were observed between elderly and younger patients.
Hepatic impairment
Ticagrelor is metabolized by the liver and impaired hepatic function can increase risks for bleeding and other adverse events. Avoid use of Ticagrelor in patients with severe hepatic impairment. There is limited experience with Ticagrelor in patients with moderate hepatic impairment; consider the risks and benefits of treatment, noting the probable increase in exposure to Ticagrelor. No dosage adjustment is needed in patients with mild hepatic impairment.
Renal impairment
No dosage adjustment is needed in patients with renal impairment. Patients receiving dialysis have not been studied.
Contraindications
History of intracranial hemorrhage
Ticagrelor is contraindicated in patients with a history of intracranial hemorrhage (ICH) because of a high risk of recurrent ICH in this population.
Active bleeding
Ticagrelor is contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage.
Hypersensitivity
Ticagrelor is contraindicated in patients with hypersensitivity (e.g., angioedema) to Ticagrelor or any component of the product.
Severe hepatic impairment
Ticagrelor is contraindicated in patients with severe hepatic impairment.
Strong CYP3A4 inhibitors
Co-administration of Ticagrelor with strong CYP3A4 inhibitors (e.g. ketoconazole, clarithromycin, nefazodone, ritonavir, and atazanavir) is contraindicated as co-administration may lead to a substantial increase in exposure to Ticagrelor.
Warnings and Precautions
Bleeding risk
The use of Ticagrelor in patients at known increased risk for bleeding should be balanced against the benefit in terms of prevention of atherothrombotic events. If clinically indicated, Ticagrelor should be used with caution in the following patient groups :
- Patients with a propensity to bleed (e.g. due to recent trauma, recent surgery, coagulation disorders, active or recent gastrointestinal bleeding). The use of Ticagrelor is contraindicated in patients with active pathological bleeding, in those with a history of intracranial haemorrhage, and in patients with severe hepatic impairment.
- Patientswith concomitant administration of medicinal products that may increase the risk of bleeding (e.g. non-steroidal anti-infammatory drugs(NSAIDs), oral anticoagulantsand/orfbrinolytics)within 24hours of Ticagrelor dosing.
Platelet transfusion did not reverse the antiplatelet effect of Ticagrelor in healthy volunteers and is unlikely to be of clinical benefit in patients with bleeding. Since co-administration of Ticagrelor with desmopressin did not decrease templatebleeding time, desmopressin is unlikely to be effective in managing clinical bleeding events. Antifbrinolytic therapy (aminocaproic acid or tranexamic acid) and/or recombinant factor Vlla therapy may increase haemostasis. Ticagrelor may be resumed after the cause of bleeding has been identifed and controlled.
Surgery
Patients should be advised to inform physicians and dentists that they are taking Ticagrelor before any surgery is scheduled and before any new medicinal product is taken.
Patients with prior ischaemic stroke
ACS patients with prior ischaemic stroke can be treated with Ticagrelor for up to 12 months.
Hepatic impairment
Use of Ticagrelor is contraindicated in patients with severe hepatic impairment. There is limited experience with Ticagrelor
in patients with moderate hepatic impairment, therefore, caution is advised in these patients.
Patients at risk for bradycardic events
Due to the limited clinical experience, Ticagrelor should be used with caution in patients with an increased risk of bradycardic events (e.g. patients without a pacemaker who have sick sinus syndrome, 2nd or 3rd degree A V block or bradycardic-related syncope).
In addition, caution should be exercised when administering Ticagrelor concomitantly with medicinal products known to induce bradycardia.
Dyspnea
Dyspnea was reported in patients treated with Ticagrelor. Dyspnoea is usually mild to moderate in intensity and often resolves without need for treatment discontinuation. Patients with asthma/chronic obstructive pulmonary disease (COPD) may have an increased absolute risk of experiencing dyspnoea with Ticagrelor. Ticagrelor should be used with caution in patients with history of asthma and/or COPD. The mechanism has not been elucidated. If a patient reports new, prolonged or worsened dyspnoea this should be investigated fully and if not tolerated, treatment with Ticagrelor should be stopped.
Creatinine elevations
Creatinine levels may increase during treatment with Ticagrelor. The mechanism has not been elucidated. Renal function should be checked according to routine medical practice. In patients with ACS, it is recommended that renal function is also checked one month after initiating the treatment with Ticagrelor, paying special attention to patients ≥ 75 years, patients with moderate/severe renal impairment and those receiving concomitant treatment with an angiotensin receptor blocker (ARB).
Uric acid increase
Hyperuricaemia may occur during treatment with Ticagrelor. Caution is advised in patients with history of hyperuricaemia or gouty arthritis. As a precautionary measure, the use of Ticagrelor in patients with uric acid nephropathy is discouraged.
Premature discontinuation
Premature discontinuation with any antiplatelet therapy, including Ticagrelor, could result in an increased risk of cardiovascular (CV) death, MI or stroke due to the patient’s underlying disease. Therefore, premature discontinuation of treatment should be avoided.
Drug interactions
Ticagrelor is primarily a CYP3A4 substrate and a mild inhibitor of CYP3A4. Ticagrelor is also a P-glycoprotein (P-gp) substrate and a weak P-gp inhibitor and may increase the exposure of P-gp substrates.
Effects of medicinal and other products on Ticagrelor
CYP3A4 inhibitors
- Strong CYP3A4 inhibitors - Strong CYP3A inhibitors substantially increase Ticagrelor exposure and so increase the risk of dyspnea, bleeding and other adverse events. Avoid use of strong inhibitors of CYP3A (e.g., ketoconazole, itraconazole, voriconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfnavir, indinavir, atazanavir and telithromycin).
- Moderate CYP3A4 inhibitors - There was no effect of Ticagrelor on diltiazem plasma levels. Other moderate CYP3A4 inhibitors (e.g. amprenavir, aprepitant, erythro-mycin and fuconazole) would be expected to have a similar effect and can as well be co-administered with Ticagrelor
- A2-fold increase of Ticagrelor exposure was observed after daily consumption of large quantities of grapefruit juice (3x200 ml). This magnitude of increased exposure is not expected to be clinically relevant to most patients.
Strong CYP3A inducers
Strong CYP3A inducers substantially reduce Ticagrelor exposure and so decrease the efficacy of Ticagrelor. Avoid use with strong inducers of CYP3A (e.g., rifampin, phenytoin, carbamazepine and phenobarbital).
Cyolosoorine (P-go and CYP3A inhibitor) Co-administration of cyclosporine (600 mg) with Ticagrelor increased Ticagrelor Cmax and AUG equal to 2.3-fold and 2.8-fold, respectively. The AUC of the active metabolite was increased by 32% and Cmax was decreased by 15% in the presence of cyclosporine.
No data are available on concomitant use of Ticagrelor with other active substances that also are potent P-gp inhibitors and moderate CYP3A4 inhibitors (e.g. verapamil, quinidine) that also may increase Ticagrelor exposure. If the association cannot be avoided, their concomitant use should be made with caution.
Others
Clinical pharmacology interaction studies showed that co-administration of Ticagrelor with heparin, enoxaparin and ASA or desmopressin did not have any effect on the pharmacokinetics of Ticagrelor or the active metabolite or on ADP-induced platelet aggregation compared with Ticagrelor alone. If clinically indicated, medicinal products that alter haemostasis should be used with caution in combination with Ticagrelor. A delayed and decreased exposure to oral P2Y12 inhibitors, including Ticagrelor and its active metabolite, has been observed in patients with ACS treated with morphine (35% reduction in Ticagrelor exposure). This interaction may be related to reduced gastrointestinal motility and apply to other opioids. The clinical relevance is unknown, but data indicate the potential for reduced Ticagrelor efficacy in patients co-administered Ticagrelor and morphine. In patients with ACS, in whom morphine cannot be withheld and fast P2Y12 inhibition is deemed crucial, the use of a parenteral P2Y12 inhibitor may be considered.
Effects of Ticagrelor on other medicinal products
Medicinal products metabolised by CYP3A4
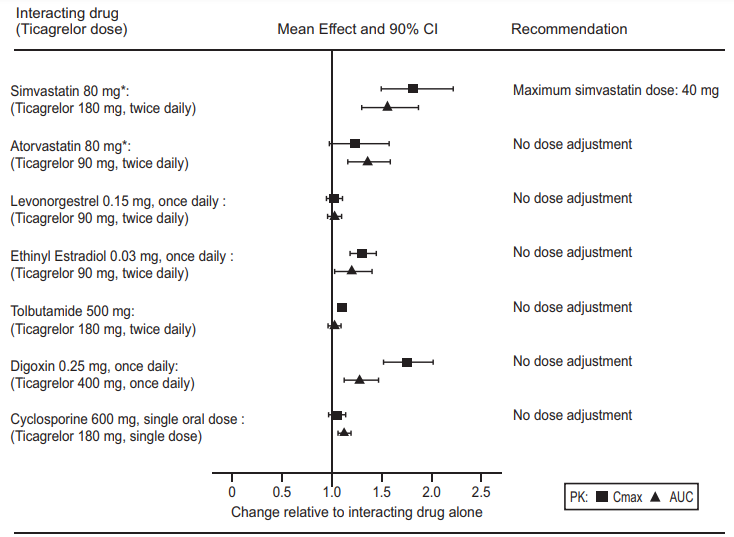
• Simvastatin and Lovastatin - Ticagrelor increases serum concentrations of simvastatin and lovastatin because these drugs are metabolized by CYP3A4. Avoid simvastatin and lovastatin doses greater than 40 mg.
• Atorvastatin - Co-administration of atorvastatin and Ticagrelor increased atorvastatin acid Cmax by 23% and AUC by 36%. Similar increases in AUC and Cmax were observed for all atorvastatin acid metabolites. These increases are not considered clinically signifcant.
• A similar effect on other statins metabolised by CYP3A4 cannot be excluded.
Ticagrelor is a mild CYP3A4 inhibitor. Co-administration of Ticagrelor and CYP3A4 substrates with narrow therapeutic indices (i.e. cisapride or ergot alkaloids) is not recommended, as Ticagrelor may increase the exposure to these medicinal products.
P-gp substrates (including digoxin, cyclosporine)
Concomitant administration of Ticagrelor increases the serum concentrations of the digoxin. In the presence of digoxin, the Cmax and AUC of Ticagrelor and its active metabolite were not affected. Therefore, appropriate clinical and/or laboratory monitoring is recommended when giving narrow therapeutic index P-gp dependent medicinal products like digoxin concomitantly with Ticagrelor. There was no effect of Ticagrelor on cyclosporine blood levels. Effect of Ticagrelor on other P-gp substrates has not been studied
Medicinal products metabolised by CYP2C9 Co-administration of Ticagrelor with tolbutamide resulted in no change in the plasma levels of either medicinal product, which suggests that Ticagrelor is not a CYP2C9 inhibitor and unlikely to alter the CYP2C9 mediated metabolism of medicinal products like warfarin and tolbutamide
Oral contraceptives
Co-administration of Ticagrelor and levonorgestrel and ethinyl estradiol increased ethinyl estradiol exposure approximately 20% but did not alter the pharmacokinetics of levonorgestrel. No clinically relevant effect on oral contraceptive effcacy is expected when levonorgestrel and ethinyl estradiol are co-administered with Ticagrelor.
Medicinal products known to induce bradvcardia
Due to observations of mostly asymptomatic ventricular pauses and bradycardia, caution should be exercised when administering Ticagrelor concomitantly with medicinal products known to induce bradycardia.
Other concomitant therapy
Co-administration of Ticagrelor with heparin, enoxaparin or desmopressin had no effect on activated partial thromboplastin time (aPTT), activated coagulation time (ACT) or factor Xa assays. However, due to potential pharmacodynamic interactions, caution should be exercised with the concomitant administration of Ticagrelor with medicinal products known to alter haemostasis
Due to reports of cutaneous bleeding abnormalities with SSRIs (e.g. paroxetine, seffraline and citalopram), caution is advised when administering SSRIs with Ticagrelor as this may increase the risk of bleeding.
Undesirable Effects
The following adverse reactions have been identifed following studies or
have been reported in post-marketing experience with Ticagrelor.
Adverse reactions are listed by MedDRA and System Organ Class (SOC).
Within each SOC the adverse reactions are ranked by frequency category.
Frequency categories are defined according to the following conventions:
Very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100),
rare (≥1/10,000 to <1/1,000), very rare (<1/10,000), not known
(cannot be estimated from the available data).
Table 1 : Adverse reactions by frequency and system organ class (SOC)
| SOC | Very common | Common | Uncommon | Not Known |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | Tumour bleedings | |||
| Blood and lymphatic system disorders | Blood disorder bleedings |
Thrombotic thrombocy-topenic purpura |
||
| Immune system disorders | Hypersensitivity including angioedema | |||
| Metabolism and nutrition disorders | Hyperuricaemia | Gout/Gouty Arthritis | ||
| Psychiatric disorders | Confusion | |||
| Nervous system disorders | Dizziness, Syncope, Headache | Intracranial haemorrhage | ||
| Eye disorders | Eye haemorrhage | |||
| Ear and labyrinth disorders | Vertigo | Ear haemorrhage | ||
| Vascular disorders | Hypotension | |||
| Respiratory, thoracic and mediastinal disorders | Dyspnoea | Respiratory system bleedings | ||
| Gastrointestinal disorders | Gastrointestinal haemorrhage, Diarrhoea, Nausea, Dyspepsia, Constipation | Retroperitoneal haemorrhage | ||
| Skin and subcutaneous tissue disorders | Subcutaneous or dermal bleeding, Rash, Pruritus | |||
| Musculoskeletal connective tissue and bone | Muscular bleedings | |||
| Renal and urinary disorders | Urinary tract bleeding | |||
| Reproductive system and breast disorders | Reproductive system bleedings | |||
| Investigations | Blood creatinine increased | |||
| Injury, poisoning and procedural complications | Post procedural haemorrhage, Traumatic bleeding |
Overdosage
There is currently no known treatment to reverse the effects of Ticagrelor, and Ticagrelor is not expected to be dialyzable. Treatment of overdose should follow local standard medical practice. Bleeding is the expected pharmacologic effect of overdosing. If bleeding occurs, appropriate supportive measures should be taken. Platelet transfusion did not reverse the antiplatelet effect of Ticagrelor in healthy volunteers and is unlikely to be of clinical benefit in patients with bleeding. Other effects of overdose may include gastrointestinal effects (nausea, vomiting, and diarrhea) or ventricular pauses. Monitor the ECG.
Pharmacodynamics and Pharmacokinetics
Mechanism of action
Ticagrelor and its major metabolite reversibly interact with the platelet P2Y12 ADP-receptor to prevent signal transduction and platelet activation. Ticagrelor and its active metabolite are approximately equipotent
Pharmacodynamics
The inhibition of platelet aggregation (IPA) by Ticagrelor and clopidogrel was compared in a 6-week study examining both acute and chronic platelet inhibition effects in response to 20 μm ADP as the platelet aggregation agonist. The onset of IPA was evaluated on day 1 of the study following loading doses of 180 mg Ticagrelor or 600 mg clopidogrel. As shown in figure 2, IPA was higher in the Ticagrelor group at all-time points. The maximum IPA effect of Ticagrelor was reached at around 2 hours, and was maintained for at least 8 hours. The offset of IPA was examined after 6 weeks on Ticagrelor 90 mg twice daily or clopidogrel 75 mg daily, again in response to 20 µm ADP.
As shown in figure 3, mean maximum IPA following the last dose of Ticagrelor was 88% and 62% for clopidogrel. The insert in figure 3 shows that after 24 hours, IPA in the Ticagrelor group (58%) was similar to IPA in clopidogrel group (52%), indicating that patients who miss a dose of Ticagrelor would still maintain IPA similar to the trough IPA of patients treated with clopidogrel. After 5 days, IPA in the Ticagrelor group was similar to IPA in the placebo group. It is not known how either bleeding risk or thrombotic risk track with IPA, for either Ticagrelor or clopidogrel.
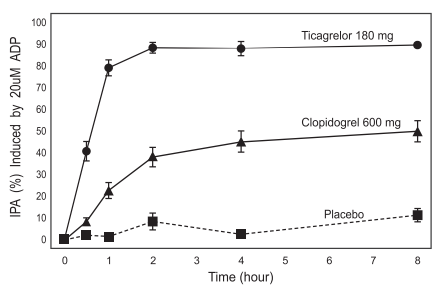
Figure 2 : Mean inhibition of platelet aggregation (±se) following single oral doses of placebo, 180 mg Ticagrelor or 600 mg clopidogrel.
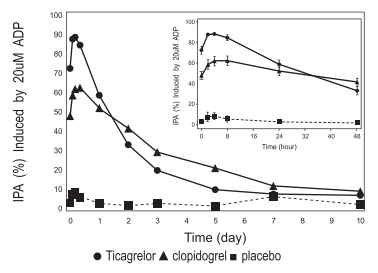
Figure 3 : Mean inhibition of platelet aggregation (IPA) following 6 weeks on placebo, Ticagrelor 90 mg twice daily, or clopidogrel 75 mg daily.
Transitioning from clopidogrel to Ticagrelor resulted in an absolute IPA increase of 26.4% and from Ticagrelor to clopidogrel resulted in an absolute IPA decrease of 24.5%. Patients can be transitioned from clopidogrel to Ticagrelor without interruption of antiplatelet effect.
Pharmacokinetics
Absorption
Ticagrelor can be taken with or without food. Absorption of Ticagrelor occurs with a median tmax of 1.5 h (range 1.0 - 4.0). The formation of the major circulating metabolite AR-C124910XX (active) from Ticagrelor occurs with a median tmax of 2.5 h (range 1.5 - 5.0).
The mean absolute bioavailability of Ticagrelor is about 36% (range 30% - 42%). Ingestion of a high-fat meal had no effect on Ticagrelor Cmax, but resulted in a 21% increase in AUC. The Cmax of its major metabolite was decreased by 22% with no change in AUC.
Ticagrelor as crushed tablets mixed in water, given orally or administered through a nasogastric tube into the stomach, is bioequivalent to whole tablets (AUC and Cmax within 80 - 125% for Ticagrelor and AR-C124910XX) with a median tmax of 1.0 hour (range 1.0 - 4.0) for Ticagrelor and 2.0 hours (range 1.0 - 8.0) for AR C124910XX.
Distribution.
The steady state volume of distribution of Ticagrelor is 88 L. Ticagrelor and the active metabolite are extensively bound to human plasma proteins (>99%).
Metabolism
CYP3A4 is the major enzyme responsible for Ticagrelor metabolism and the formation of its major active metabolite. Ticagrelor and its major active metabolite are weak p-glycoprotein substrates and inhibitors. The systemic exposure to the active metabolite is approximately 30 - 40% of the exposure of Ticagrelor.
Excretion
The primary route of Ticagrelor elimination is hepatic metabolism. When radiolabeled Ticagrelor is administered, the mean recovery of radioactivity is approximately 84% (58% in feces, 26% in urine). Recoveries of Ticagrelor and the active metabolite in urine were both less than 1% of the dose. The primary route of elimination for the major metabolite of Ticagrelor is most likely to be biliary secretion. The mean t is approximately 7 hours for Ticagrelor and 9 hours for the 1/2 active metabolite.
Specifc populations
The effects of age, gender, ethnicity, renal impairment and mild hepatic impairment on the pharmacokinetics of Ticagrelor are presented in figure 4. Effects are modest and do not require dose adjustment.
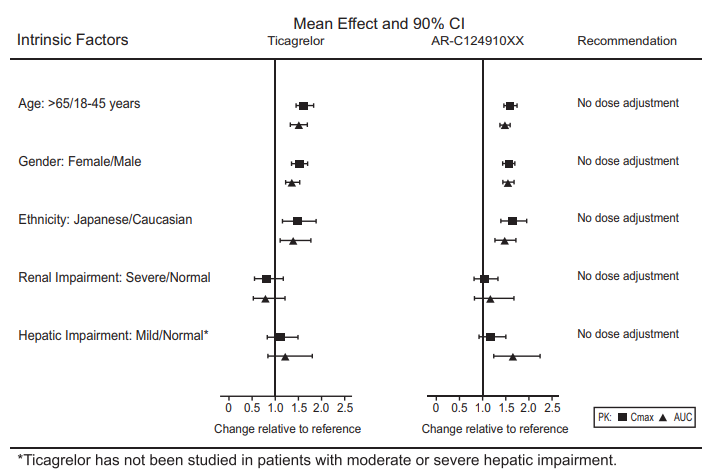
Figure 4 : Impact of intrinsic factors on the pharmacokinetics of Ticagrelor
Effects of other drugs on Ticagrelor
CYP3A4 is the major enzyme responsible for Ticagrelor metabolism and the formation of its major active metabolite. The effects of other drugs on the pharmacokinetics of Ticagrelor are presented in figure 5 as change relative to Ticagrelor given alone (test/reference). Strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, and clarithromycin) substantially increase Ticagrelor exposure. Moderate CYP3A inhibitors have lesser effects (e.g., diltiazem). CYP3A inducers (e.g., rifampin) substantially reduce Ticagrelor blood levels. P-gp inhibitors (e.g., cyclosporine) increase Ticagrelor exposure. Co-administration of 5 mg intravenous morphine with 180 mg loading dose of Ticagrelor decreased observed mean Ticagrelor exposure by up to 25% in healthy adults and up to 36% in ACS patients undergoing PCI. Tmax was delayed by 1-2 hours. Exposure of the active metabolite decreased to a similar extent. Morphine co-administration did not delay or decrease platelet inhibition in healthy adults. Mean platelet aggregation was higher up to 3 hours post loading dose in ACS patients co-administered with morphine. Co-administration of intravenous fentanyl with 180 mg loading dose of Ticagrelor in ACS patients undergoing PCI resulted in similar effects on Ticagrelor exposure and platelet inhibition.
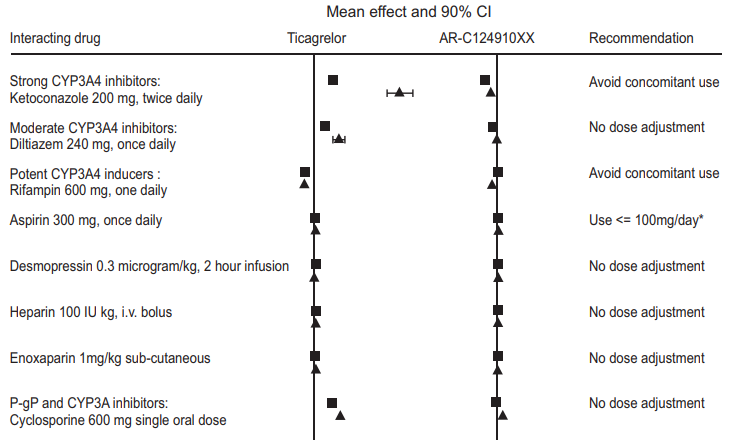
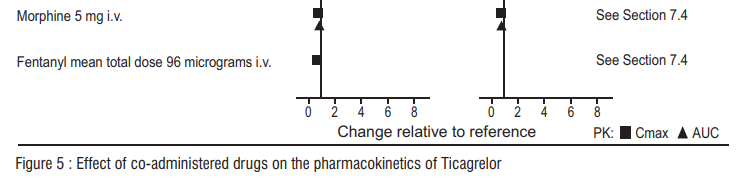
Effects of Ticagrelor on other drugs
In vitro metabolism studies demonstrate that Ticagrelor and its major active metabolite are weak inhibitors of CYP3A4, potential activators of CYP3A5 and inhibitors of the p-gp transporter. Ticagrelor and AR-C124910XX were shown to have no inhibitor y effect on human CYP1A2, CYP2C19, and CYP2E1 activity. For specifc in vivo effects on the Pharmacokinetics of simvastatin, atorvastatin, ethinyl estradiol, levonorgesterol, tolbutamide, digoxin and cyclosporine, see figure 6.

Shelf-life
Refer on the pack
Packing information
Alu-Alu blister strip of 10 tablets.
Storage
Store protected from light and moisture, at a temperature not exceeding 30°C
Keep out of reach of children.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What is Ticabest and what it is used for
2. What you need to know before you take Ticabest
3. How to take Ticabest
4. Possible side effects
5. How to store Ticabest
6. Contents of the pack and other information
1. What is Ticabest and What It is Used for
What Ticabest is
Ticabest contains an active substance called ticagrelor. This belongs to a group of medicines called antiplatelet medicines.
What Ticabest is used for
Ticabest in combination with acetylsalicylic acid (another antiplatelet agent) is to be used in adults only. You have been given this medicine because you have had:
- a heart attack, or
- unstable angina (angina or chest pain that is not well controlled).
It reduces the chances of you having another heart attack, stroke or dying from a disease related to your heart or blood vessels.
How Ticabest works
Ticabest affects cells called ‘platelets’ (also called thrombocytes). These very small blood cells help stop bleeding by clumping together to plug tiny holes in blood vessels that are cut or damaged.
However, platelets can also form clots inside diseased blood vessels in the heart and brain. This can be very dangerous because:
- the clot can cut off the blood supply completely; this can cause a heart attack (myocardial infarction) or stroke, or
- the clot can partly block the blood vessels to the heart; this reduces the blood flow to the heart and can cause chest pain which comes and goes (called ‘unstable angina’).
Ticabest helps stop the clumping of platelets. This reduces the chance of a blood clot forming that can reduce blood flow.
2. What You Need to Know Before You Take Ticabest
Do not take Ticabest if
You are allergic to ticagrelor or any of the other ingredients of this medicine listed in the formulation.
You are bleeding now.
You have had a stroke caused by bleeding in the brain.
You have severe liver disease.
You are taking any of the following medicines:
- ketoconazole (used to treat fungal infections)
- clarithromycin (used to treat bacterial infections)
- nefazodone (an antidepressant)
- ritonavir and atazanavir (used to treat HIV infection and AIDS)
Do not take Ticabest if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist before taking this medicine.
Warnings and precautions
Talk to your doctor or pharmacist before taking Ticabest if:
You have an increased risk of bleeding because of:
a recent serious injury
recent surgery (including dental work, ask your dentist about this) you have a condition that affects blood clotting - recent bleeding from your stomach or gut (such as a stomach ulcer or colon ‘polyps’)
You are due to have surgery (including dental work) at any time while taking Ticabest. This is because of the increased risk of bleeding. Your doctor may want you to stop taking this medicine 5 days prior to surgery.
Your heart rate is abnormally low (usually lower than 60 beats per minute) and you do not already have in place a device that paces your heart (pacemaker).
You have asthma or other lung problems or breathing difficulties.
You have had any problems with your liver or have previously had any disease which may have affected your liver.
You have had a blood test that showed more than the usual amount of uric acid
If any of the above apply to you (or you are not sure), talk to your doctor or pharmacist before taking this medicine.
If you are taking both Ticabest and heparin:
Your doctor may require a sample of your blood for diagnostic tests if they suspect a rare platelet disorder caused by heparin. It is important that you inform your doctor that you are taking both Ticabest and heparin, as Ticabest may affect the diagnostic test.
Children and adolescents
Ticabest is not recommended for children and adolescents under 18 years.
Other medicines and Ticabest
Please tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This is because Ticabest can affect the way some medicines work and some medicines can have an effect on Ticabest.
Tell your doctor or pharmacist if you are taking any of the following medicines:
- more than 40 mg daily of either simvastatin or lovastatin (medicines used to treat high cholesterol)
- rifampicin (an antibiotic)
- phenytoin, carbamazepine and phenobarbital (used to control seizures)
- digoxin (used to treat heart failure)
- cyclosporine (used to lessen your body’s defenses)
- quinidine and diltiazem (used to treat abnormal heart rhythms)
- beta blockers and verapamil (used to treat high blood pressure)
- morphine and other opioids (used to treat severe pain)
In particular, tell your doctor or pharmacist if you are taking any of the following medicines that increase your risk of bleeding:
oral anticoagulants’ often referred to as ʽblood thinners’ which include warfarin.
Non-Steroidal Anti-Inflammatory Drugs (abbreviated as NSAIDs) often taken as painkillers such as ibuprofen and naproxen.
Selective Serotonin Reuptake Inhibitors (abbreviated as SSRIs) taken as antidepressants such as paroxetine, sertraline and citalopram.
other medicines such as ketoconazole (used to treat fungal infections), clarithromycin (used to treat bacterial infections), nefazodone (an antidepressant), ritonavir and atazanavir (used to treat HIV infection and AIDS), cisapride (used to treat heartburn), ergot alkaloids (used to treat migraines and headaches).
Also tell your doctor that because you are taking Ticabest, you may have an increased risk of bleeding if your doctor gives you fibrinolytics, often called ‘clot dissolvers’, such as streptokinase or alteplase.
Pregnancy and breast-feeding
It is not recommended to use Ticabest if you are pregnant or may become pregnant. Women should use appropriate contraceptive measures to avoid pregnancy while taking this medicine. Talk to your doctor before taking this medicine if you are breast-feeding. Your doctor will discuss with you the benefits and risks of taking Ticabest during this time. If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Ticabest is not likely to affect your ability to drive or use machines. If you feel dizzy or confused while taking this medicine, be careful while driving or using machines.
3. How to Take Ticabest
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
How much to take
The starting dose is two tablets at the same time (loading dose of 180 mg). This dose will usually be given to you in the hospital. After this starting dose, the usual dose is one tablet of 90 mg twice a day for up to 12 months unless your doctor tells you differently. Take this medicine around the same time every day (for example, one tablet in the morning and one in the evening).
Taking Ticabest with other medicines for blood clotting
Your doctor will usually also tell you to take acetylsalicylic acid. This is a substance present in many medicines used to prevent blood clotting. Your doctor will tell you how much to take (usually between 75-150 mg daily).
How to take Ticabest
You can take the tablet with or without food.
You can check when you last took a tablet of Ticabest by looking on the blister. There is a sun (for the morning) and a moon (for the evening). This will tell you whether you have taken the dose.
If you have trouble swallowing the tablet
- If you have trouble swallowing the tablet you can crush it and mix with water as follows:
- Crush the tablet to a fine powder.
- Pour the powder into half a glass of water.
- Stir and drink immediately.
- To make sure there is no medicine left, rinse the empty glass with another half a glass of water and drink it.
If you are in the hospital you may be given this tablet mixed with some water and given through a tube via the nose (nasogastric tube).
If you take more Ticabest than you should
If you take more Ticabest than you should, talk to a doctor or go to hospital straight away. Take the medicine pack with you. You may be at increased risk of bleeding.
If you forget to take Ticabest
- If you forget to take a dose, just take your next dose as normal.
- Do not take a double dose (two doses at the same time) to make up for the forgotten dose.
If you stop taking Ticabest
Do not stop taking Ticabest without talking to your doctor. Take this medicine on a regular basis and for as long as your doctor keeps prescribing it. If you stop taking Ticabest, it may increase your chances of having another heart attack or stroke or dying from a disease related to your heart or blood vessels. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may happen with this medicine: Ticabest affects blood clotting, so most side effects are related to bleeding. Bleeding may occur in any part of the body. Some bleeding is common (like bruising and nosebleeds). Severe bleeding is uncommon but can be life threatening. See a doctor straight away if you notice any of the following – you may need urgent medical treatment:
Bleeding into the brain or inside the skull is an uncommon side effect, and may cause signs of a stroke such as:
- sudden numbness or weakness of your arm, leg or face, especially if only on one side of the body
- sudden confusion, difficulty speaking or understanding others
- sudden difficulty in walking or loss of balance or co-ordination
- suddenly feeling dizzy or sudden severe headache with no known cause
Signs of bleeding such as:
- bleeding that is severe or that you cannot control
- unexpected bleeding or bleeding that lasts a long time pink, red or brown urine
- vomiting red blood or your vomit looks like ‘coffee grounds’
- red or black stools (look like tar)
- coughing up or vomiting blood clots
Fainting (syncope)
- a temporary loss of consciousness due to sudden drop in blood flow to the brain (common)
Signs of a blood clotting problem called Thrombotic Thrombocytopenic Purpura (TTP) such as:
fever and purplish spots (called purpura) on the skin or in the mouth, with or without yellowing of the skin or eyes (jaundice), unexplained extreme tiredness or confusion.
Discuss with your doctor if you notice any of the following:
Feeling short of breath - this is very common. It might be due to your heart disease or another cause, or it might be a side effect of Ticabest. Ticabest-related breathlessness is generally mild and characterised as a sudden, unexpected hunger for air usually occurring at rest and may appear in the first weeks of therapy and for many may disappear. If you’re feeling of shortness of breath gets worse or lasts a long time, tell your doctor. Your doctor will decide if it needs treatment or further investigations.
Other possible side effects
Very common (may affect more than 1 in 10 people)
- High level of uric acid in your blood (as seen in tests)
- Bleeding caused by blood disorders
Common (may affect up to 1 in 10 people)
- Bruising
- Headache
- Feeling dizzy or like the room is spinning
- Diarrhoea or indigestion
- Feeling sick (nausea)
- Constipation
- Rash
- Itching
- Severe pain and swelling in your joints – these are signs of gout
- Feeling dizzy or light-headed, or having blurred vision – these are signs of low blood pressure
- Nosebleed Bleeding after surgery or from cuts (for example while shaving) and wounds more than is normal Bleeding from your stomach lining (ulcer) Bleeding gums
- Nosebleed
- Bleeding after surgery or from cuts (for example while shaving) and wounds more than is normal
- Bleeding from your stomach lining (ulcer)
- Bleeding gums
Uncommon (may affect up to 1 in 100 people)
- Allergic reaction – a rash, itching or a swollen face or swollen lips/tongue may be signs of an allergic reaction
- Confusion
- Visual problems caused by blood in your eye
- Vaginal bleeding that is heavier, or happens at different times, than your normal period (menstrual) bleeding
- Bleeding into your joints and muscles causing painful swelling
- Blood in your ear Internal bleeding, this may cause dizziness or light-headedness
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to Store Ticabest
Store below 300C. Protect from light and moisture
Keep out of reach of children.
6. Contents of the Pack and Other Information
What Ticabest contains
The active substance is ticagrelor. Each film-coated tablet contains 90 mg of ticagrelor.