Tosiban 6.75mg Injection
Therapy Area
Women's Health
1.0 Generic Name
Atosiban Acetate Solution for Injection (7.5mg/ml)
Atosiban Acetate Concentrate for Solution for Infusion (7.5mg/ml)
2.0 Qualitative and quantitative composition
Tosiban 6.75 mg Solution for Injection
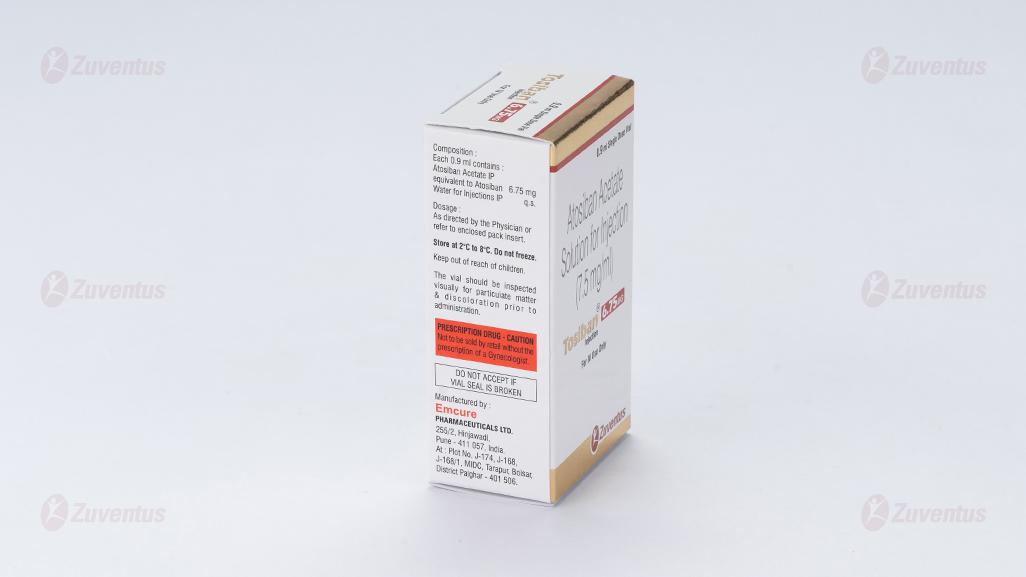
Each 0.9 ml contains:
Atosiban Acetate IP equivalent to Atosiban 6.75 mg
Water for Injections IP q.s.
Tosiban® 37.5 mg concentrate for solution for infusion
Each 5 ml contains:
Atosiban acetate IP equivalent to Atosiban 37.5 mg
Water for injection IP q.s
3.0 Dosage form and strength
Solution for injection and Infusion (For IV Use only).
7.5 mg/ml
4.0 Clinical particulars
4.1. Therapeutic indication
Tosiban® is indicated to delay imminent pre-term birth in pregnant adult women with:
- regular uterine contractions of at least 30 seconds duration at a rate of ≥ 4 per 30 minutes
- a cervical dilation of 1 to 3 cm (0-3 for nulliparas) and effacement of ≥ 50%
- a gestational age from 24 until 33 completed weeks
- a normal foetal heart rate
4.2. Posology and method of administration
Posology
Treatment with Tosiban® should be initiated and maintained by a physician experienced in the treatment of pre-term labour.
Tosiban® is administered intravenously in three successive stages: an initial bolus dose (6.75 mg), performed with Tosiban® 6.75 mg/0.9 ml solution for injection, immediately followed by a continuous high dose infusion (loading infusion 300 micrograms/min) of Tosiban® 37.5 mg/5 ml concentrate for solution for infusion during three hours, followed by a lower dose of Tosiban® 37.5 mg/5 ml concentrate for solution for infusion (subsequent infusion 100 micrograms/min) up to 45 hours. The duration of the treatment should not exceed 48 hours. The total dose given during a full course of Tosiban® therapy should preferably not exceed 330.75 mg of Tosiban®.
Intravenous therapy using the initial bolus injection should be started as soon as possible after diagnosis of pre-term labour. Once the bolus has been injected, proceed with the infusion. In the case of persistence of uterine contractions during treatment with Tosiban®, alternative therapy should be considered. The following table shows the full posology of the bolus injection followed by the infusion:
Methods of administration
Step 1: Preparation of the initial intravenous injection (Bolus):
Give a bolus dose directly intravenously using 1ml syringe. Withdraw 0.9 ml of Tosiban 6.75 mg Solution for Injection and administer slowly as an intravenous bolus dose over one minute, under adequate medical supervision in an obstetric unit.
Step 2 and 3 Method of administration:
Preparation of the intravenous infusion solution: For intravenous infusion, Tosiban injection 37.5 mg/5 ml, concentrate for solution for infusion should be diluted in one of the following solutions:
- Sodium Chloride 9 mg/ml (0.9%) Solution for Injection
- Ringer's lactate solution
- 5% w/v glucose solution.
A. Using 500 ml infusion bottles:
Preparation of the intravenous infusion solution when given in 500 ml Infusion Bottle: Withdraw 7.5 ml solution from a 500 ml infusion bottle & discard. Replace it with 7.5 ml Tosiban solution for infusion (1.5 x 5 ml vials). Remaining half vial of Tosiban (2.5 ml) to be stored at 2°C to 8°C and used for preparation of subsequent infusion.
B. Using 100ml infusion bottles
Preparation of the intravenous infusion solution when given in 100 ml Infusion Bottle/Bottle: Withdraw 7.5 ml solution from a 100 ml infusion bottle and discard. Replace it by 7.5 ml of Atosiban 37.5 mg/5 ml concentrate for solution for infusion.
Remaining half vial of Tosiban (2.5 ml) to be stored at 2°C to 8°C & used for preparation of subsequent infusion.
Re-treatment:
In case a re-treatment with Tosiban® is needed, it should also commence with a bolus injection of Tosiban® 6.75 mg/0.9 ml, solution for injection followed by infusion with Tosiban® 37.5 mg/5 ml, concentrate for solution for infusion.
Patients with renal or hepatic impairment
There is no experience with Tosiban® treatment in patients with impaired function of the liver or kidneys.
Renal impairment is not likely to warrant a dose adjustment, since only a small extent of Tosiban® is excreted in the urine. In patients with impaired hepatic function, Tosiban® should be used with caution.
Paediatric population
The safety and efficacy of Tosiban® in pregnant women aged less than 18 years have not been established. No data are available.
4.3. Contraindications
Tosiban® must not be used in the following conditions:
- Gestational age below 24 or over 33 completed weeks
- Premature rupture of the membranes >30 weeks of gestation
- Abnormal foetal heart rate
- Antepartum uterine haemorrhage requiring immediate delivery
- Eclampsia and severe pre-eclampsia requiring delivery
- Intrauterine foetal death
- Suspected intrauterine infection
- Placenta praevia
- Abruptio placenta
- Any other conditions of the mother or foetus, in which continuation of pregnancy is hazardous
- Hypersensitivity to the active substance(s) or to any of the excipients
4.4. Special warnings and precautions for use
When Tosiban® is used in patients in whom premature rupture of membranes cannot be excluded, the benefits of delaying delivery should be balanced against the potential risk of chorioamnionitis.
There is no experience with Tosiban® treatment in patients with impaired function of the liver or kidneys.
Renal impairment is not likely to warrant a dose adjustment, since only a small extent of Tosiban® is excreted in the urine. In patients with impaired hepatic function, Tosiban® should be used with caution.
There is only limited clinical experience in the use of Tosiban® in multiple pregnancies or the gestational age group between 24 and 27 weeks, because of the small number of patients treated. The benefit of Tosiban® in these subgroups is therefore uncertain.
Re-treatment with Tosiban® is possible, but there is only limited clinical experience available with multiple re-treatments, up to 3 re-treatments (see section 4.2).
In case of intrauterine growth retardation, the decision to continue or reinitiate the administration of Tosiban® depends on the assessment of fetal maturity.
Monitoring of uterine contractions and fetal heart rate during administration of Tosiban® and in case of persistent uterine contractions should be considered.
As an antagonist of oxytocin, Tosiban® may theoretically facilitate uterine relaxation and postpartum bleeding therefore blood loss after delivery should be monitored. However, inadequate uterus contraction postpartum was not observed during the clinical trials.
Multiple pregnancy and medicinal products with tocolytic activity like calcium channel blockers and betamimetics are known to be associated with increased risk of pulmonary oedema. Therefore, Tosiban® should be used with caution in case of multiple pregnancy and/or concomitant administration of other medicinal products with tocolytic activity.
4.5. Drugs interactions
It is unlikely that atosiban is involved in cytochrome P450 mediated drug-drug interactions as in vitro investigations have shown that atosiban is not a substrate for the cytochrome P450 system, and does not inhibit the drug metabolising cytochrome P450 enzymes.
Interaction studies have been performed with labetalol and betamethasone in healthy, female volunteers. No clinically relevant interaction was found between atosiban and bethamethasone or labetalol.
4.6. Fertility, pregnancy and lactation
Fertility
Embryo-fetal toxicity studies have not shown toxic effects of atosiban. No studies were performed that covered fertility and early embryonic development (see section 5.3).
Pregnancy
Tosiban® should only be used when pre-term labour has been diagnosed between 24 and 33 completed weeks of gestation. If during pregnancy the woman is already breast-feeding an earlier child, then breast-feeding should be discontinued during treatment with Tosiban®, since the release of oxytocin during breast-feeding may augment uterine contractility, and may counteract the effect of tocolytic therapy.
Breastfeeding
In Tosiban® clinical trials, no effects were observed on breast-feeding. Small amounts of Tosiban® have been shown to pass from plasma into the breast milk of breast-feeding women.
4.7. Effects on ability to drive and use machines
Not relevant.
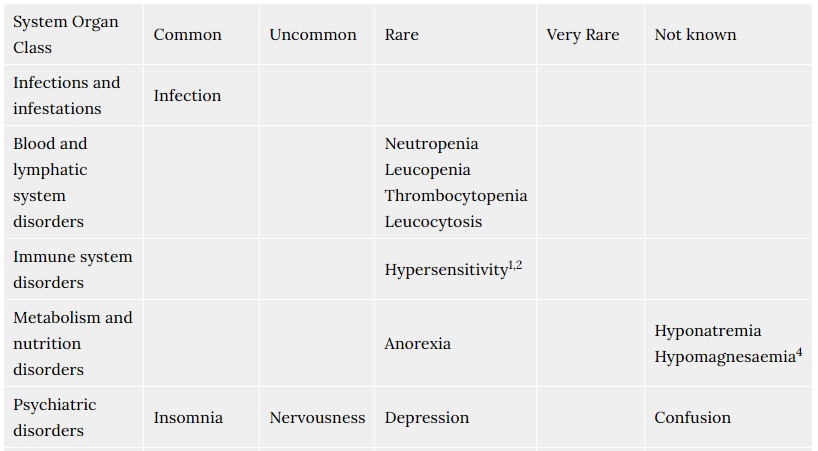
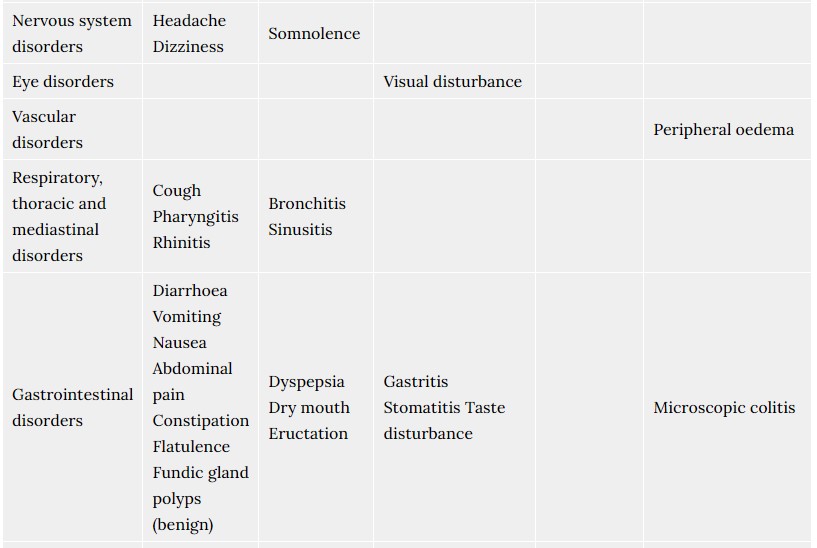
4.8. Undesirable effects
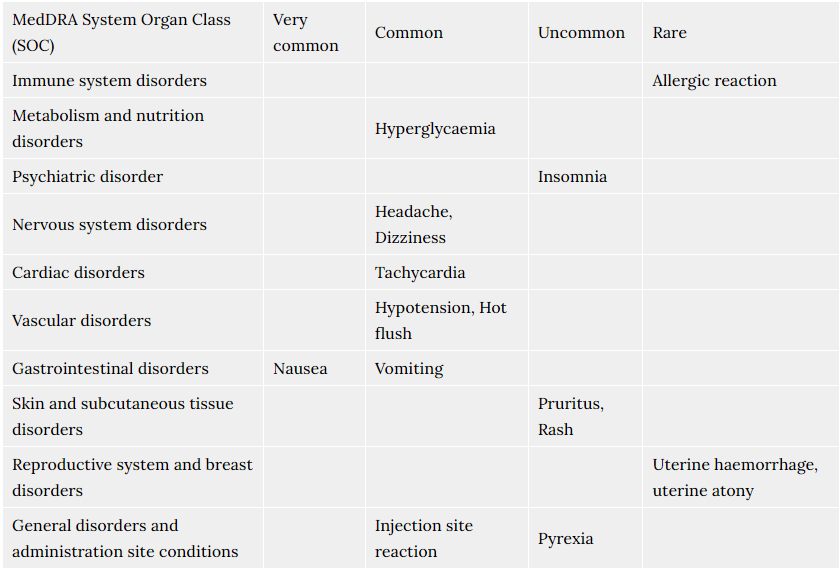
Possible adverse reactions of Tosiban® were described for the mother during the use of Tosiban® in clinical trials. In total 48% of the patients treated with Tosiban® experienced adverse reactions during the clinical trials. The observed adverse reactions were generally of a mild severity. The most commonly reported adverse reaction in the mother is nausea (14%).
For the newborn, the clinical trials did not reveal any specific adverse reactions of Tosiban®. The infant adverse reactions were in the range of normal variation and were comparable with both placebo and beta-mimetic group incidences.
The frequency of adverse reactions listed below is defined using the following convention: Very common (≥1/10); Common (≥1/100 to <1/10); Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000).
Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Post-marketing experience
Respiratory events like dyspnoea and pulmonary oedema, particularly in association with concomitant administration of other medicinal products with tocolytic activity, like calcium antagonists and beta-mimetics, and/or in women with multiple pregnancy, have been reported post-marketing.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com Website link: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9. Overdose
Few cases of Tosiban® overdosing were reported, they occurred without any specific signs or symptoms. There is no known specific treatment in case of an overdose.
5.0 Pharmacological properties
5.1 Pharmacodynamic properties
Mechanism of action
Tosiban® contains Atosiban (INN), a synthetic peptide ([Mpa1,D- Tyr(Et)2,Thr4,Orn8]-oxytocin) which is a competitive antagonist of human oxytocin at receptor level. In rats and guinea pigs, Atosiban was shown to bind to oxytocin receptors, to decrease the frequency of contractions and the tone of the uterine musculature, resulting in a suppression of uterine contractions. Atosiban was also shown to bind to the vasopressin receptor, thus inhibiting the effect of vasopressin. In animals, Atosiban did not exhibit cardiovascular effects.
Pharmacodynamic effects
In human pre-term labour, Atosiban at the recommended dosage antagonises uterine contractions and induces uterine quiescence. The onset of uterus relaxation following Atosiban is rapid, uterine contractions being significantly reduced within 10 minutes to achieve stable uterine quiescence (≤ 4 contractions/hour) for 12 hours.
5.2 Pharmacokinetic properties
Absorption
In healthy non-pregnant subjects receiving atosiban infusions (10 to 300 micrograms/min over 12 hours), the steady state plasma concentrations increased proportionally to the dose.
Distribution
The clearance, volume of distribution and half-life were found to be independent of the dose.
In women in pre-term labour receiving atosiban by infusion (300 micrograms/min for 6 to 12 hours), steady state plasma concentrations were reached within one hour following the start of the infusion (mean 442 ± 73 ng/ml, range 298 to 533 ng/ml). Following completion of the infusion, plasma concentration rapidly declined with an initial (tα) and terminal (tβ) half-life of 0.21 ± 0.01 and 1.7 ± 0.3 hours, respectively. Mean value for clearance was 41.8 ± 8.2 litres/h. Mean value of volume of distribution was 18.3 ± 6.8 litres. Plasma protein binding of atosiban is 46 to 48% in pregnant women. It is not known whether the free fraction in the maternal and fetal compartments differs substantially. Tosiban® does not partition into red blood cells.
Atosiban passes the placenta. Following an infusion of 300 micrograms/min in healthy pregnant women at term, the fetal/maternal Tosiban® concentration ratio was 0.12.
Biotransformation
Two metabolites were identified in the plasma and urine from human subjects. The ratios of the main metabolite M1 (des-(Orn8, Gly-NH29)-[Mpa1, D-Tyr(Et)2, Thr4]-oxytocin) to atosiban concentrations in plasma were 1.4 and 2.8 at the second hour and at the end of the infusion respectively. It is not known whether M1 accumulates in tissues. Atosiban is found in only small quantities in urine, its urinary concentration is about 50 times lower than that of M1. The proportion of atosiban eliminated in faeces is not known. The main metabolite M1 is approximately 10 times less potent than atosiban in inhibiting oxytocin-induced uterine contractions in vitro. Metabolite M1 is excreted in milk (see section 4.6).
Elimination
There is no experience with atosiban treatment in patients with impaired function of the liver or kidneys.
Renal impairment is not likely to warrant a dose adjustment, since only a small extent of atosiban is excreted in the urine. In patients with impaired hepatic function, atosiban should be used with caution (see sections 4.2 and 4.4).
It is unlikely that atosiban inhibits hepatic cytochrome P450 isoforms in humans (see section 4.5).
6.0 Nonclinical properties
No systemic toxic effects were observed during the two-week intravenous toxicity studies (in rats and dogs) at doses which are approximately 10 times higher than the human therapeutic dose, and during the three months toxicity studies in rats and dogs (up to 20 mg/kg/day s.c.). The highest atosiban subcutaneous dose not producing any adverse effects was approximately two times the therapeutic human dose. No studies were performed that covered fertility and early embryonic development. Reproduction toxicity studies, with dosing from implantation up to late stage pregnancy, showed no effects on mothers and fetuses.
The exposure of the rat fetus was approximately four times that received by the human fetus during intravenous infusions in women. Animal studies have shown inhibition of lactation as expected from the inhibition of action of oxytocin.
Atosiban was neither oncogenic nor mutagenic in in vitro and in vivo tests.
7.0 Description
Tosiban® contains atosiban acetate. It is a competitive antagonist of human oxytocin receptors and thus, decreases uterine contractions. Each ml of Tosiban® solution contains 7.5 mg atosiban for solution for injection or infusion.
Chemical structure of atosiban.
Molecular Formula: C43H67N11O12S2
Molecular Weight: 994.2 g/mol
8.0 Pharmaceutical particulars
8.1 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products except mentioned in this section. For intravenous infusion, following the bolus dose, Tosiban® 37.5 mg/5 ml, concentrate for solution for infusion should be diluted in one of the following solutions: - Sodium Chloride (0.9%) solution - Ringer's lactate solution - 5% w/v glucose solution.
8.2 Shelf-life
Refer on pack.
8.3 Packaging information
Tosiban 6.75 mg: A vial of 0.9 ml.
Tosiban 37.5 mg: A vial of 5 ml.
8.4 Storage and handing instructions
Store at 2°C to 8°C. Do not freeze.
Store in the original package in order to protected from light.
Once the vial has been opened, the dilution must be performed immediately. Diluted solution for intravenous administration should be used within 24 hours’ after preparation.
The vial should be inspected visually for particulate matter & discoloration prior to administration.
Keep out of reach of children.
9.0 Patient Counselling Information
Talk to your doctor or nurse before you are given Tosiban®:
- if you think your waters might have broken (premature rupture of your membranes).
- if you have kidney or liver problems.
- if you are pregnant with more than one baby.
- if your contractions start again, treatment with Tosiban® can be repeated up to three more times.
- if your unborn baby is small for the time of your pregnancy.
- Your womb may be less able to contract after your baby has been born. This may cause bleeding.
- if you are pregnant with more than one baby and/or are given medicines that can delay the birth of your baby, such as medicines used for high blood pressure. This may increase the risk of lung oedema (accumulation of fluid in the lungs).
If any of the above apply to you (or you are not sure), talk to your doctor, before you are given Tosiban®.
12.0 Date of revision
27th June 2024
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
- What TOSIBAN® is and what it is used for
- What you need to know before you take TOSIBAN®
- How to take TOSIBAN®
- Possible side effects
- How to store TOSIBAN®
- Contents of the pack and other information
1. What TOSIBAN® is and what it is used for
TOSIBAN® solution for injection contains Atosiban. TOSIBAN® can be used to delay the premature birth of your baby. TOSIBAN® is used in pregnant adult women, from week 24 to week 33 of the pregnancy. TOSIBAN® works by making the contractions in your womb (uterus) less strong. It also makes the contractions happen less often. It does this by blocking the effect of a natural hormone in your body called “oxytocin” which causes your womb (uterus) to contract.
2. What you need to know before you take TOSIBAN®
Do not take TOSIBAN®
- If you are less than 24 weeks pregnant.
- If you are more than 33 weeks pregnant.
- If your waters have broken (premature rupture of your membranes) and you have completed 30 weeks of your pregnancy or more. If your unborn baby (foetus) has an abnormal heart rate.
- If you have bleeding from your vagina and your doctor wants your unborn baby to be delivered straight away.
- If you have something called “severe pre-eclampsia” and your doctor wants your unborn baby to be delivered straight away. Severe pre-eclampsia is when you have very high blood pressure, fluid retention and/or protein in your urine.
- If you have something called “eclampsia” which is similar to “severe pre-eclampsia” but you would also have fits (convulsions). This will mean your unborn baby needs to be delivered straight away.
- If your unborn baby has died.
- If you have or could have an infection of your womb (uterus).
- If your placenta is covering the birth canal.
- If your placenta is detaching from the wall of your womb.
- If you or your unborn baby have any other conditions where it would be dangerous to continue with your pregnancy.
- If you are allergic to TOSIBAN® or any of the other ingredients.
Do not use TOSIBAN® if any of the above apply to you. If you are not sure, talk to your doctor, midwife or pharmacist before you are given TOSIBAN®.
Warnings and precautions
Talk to your doctor or pharmacist or nurse before using TOSIBAN®:
- If you think your waters might have broken (premature rupture of your membranes).
- If you have kidney or liver problems.
- If you are between 24 and 27 weeks pregnant.
- If you are pregnant with more than one baby.
- If your contractions start again, treatment with TOSIBAN® can be repeated up to three more times.
- If your unborn baby is small for the time of your pregnancy. Your womb may be less able to contract after your baby has been born. This may cause bleeding.
- If you are pregnant with more than one baby and/or are given medicines that can delay the birth of your baby, such as medicines used for high blood pressure. This may increase the risk of lung oedema (accumulation of fluid in the lungs).
- If any of the above apply to you (or you are not sure), talk to your doctor, midwife or pharmacist before you are given TOSIBAN®.
Children and adolescents
TOSIBAN® has not been studied in pregnant women less than 18 years old.
Other medicines and TOSIBAN®
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Pregnancy, breast-feeding and fertility
You should only be given this medicine if you are between 24 and 33 weeks of pregnancy. If this does not apply to you, please speak to your doctor. If you are still breast feeding a child from an earlier pregnancy, you should stop during your treatment with TOSIBAN®, as breastfeeding may stimulate uterine contractions.
3. How to use TOSIBAN®
TOSIBAN® will be given to you in a hospital by a doctor, nurse or midwife. They will decide how much you need. They will also make sure the solution is clear and free from particles.
TOSIBAN® will be given into a vein (intravenously) in three stages:
- The first injection of 6.75 mg in 0.9 ml will be slowly injected into your vein over one minute.
- Then a continuous infusion (drip) will be given at a dose of 18 mg per hour for 3 hours.
- Then another continuous infusion (drip) at a dose of 6 mg per hour will be given for up to 45 hours, or until your contractions have stopped.
Treatment should last no longer than 48 hours in total. Further treatment with TOSIBAN® can be used if your contractions start again. Treatment with TOSIBAN® can be repeated up to three more times. During treatment with TOSIBAN®, your contractions and your unborn baby’s heart rate may be monitored. It is recommended that no more than three re-treatments should be used during a pregnancy.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The side effects seen in the mother are generally of a mild severity. There are no known side effects on the unborn or new-born baby.
The following side effects may happen with this medicine:
Very common (affects more than 1 in 10 people)
- Feeling sick (nausea).
Common (affects less than 1 in 10 people)
- Headache.
- Feeling dizzy.
- Hot flushes.
- Being sick (vomiting).
- Fast heart beat.
- Low blood pressure.Signs may include feeling dizzy or light-headed.
- A reaction at the site where the injection was given.
- High blood sugar.
Uncommon (affects less than 1 in 100 people)
- High temperature (fever).
- Difficulty sleeping (insomnia).
- Itching.
- Rash.
Rare (affects less than 1 in 1,000 people)
- Your womb may be less able to contract after your baby has been born. This may cause bleeding.
- Allergic reactions.
You may experience shortness of breath or lung oedema (accumulation of fluid in the lungs), particularly if you are pregnant with more than one baby and/or are given medicines that can delay the birth of your baby, such as medicines used for high blood pressure.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store TOSIBAN®
Keep this medicine out of the sight and reach of children. Do not use TOSIBAN® after the expiry date (EXP:) which is stated on the pack. The expiry date refers to the last day of that month. Store in a refrigerator (2°C - 8°C). Store in the original package in order to protect from light. Once the ampoule has been opened, the product must be used straight away. Do not use this medicine if you notice particulate matter and discoloration prior to administration.
6. Contents of the pack and other information
What TOSIBAN® contains
Tosiban 6.75 mg Solution for Injection
Each 0.9 ml contains
Atosiban Acetate IP equivalent to Atosiban 6.75 mg
Water for Injections IP q.s.
Tosiban 37.5 mg Concentrate for Solution for Infusion
Each 5 ml contains
Atosiban Acetate IP equivalent to Atosiban 37.5 mg
Water for Injections IP q.s.