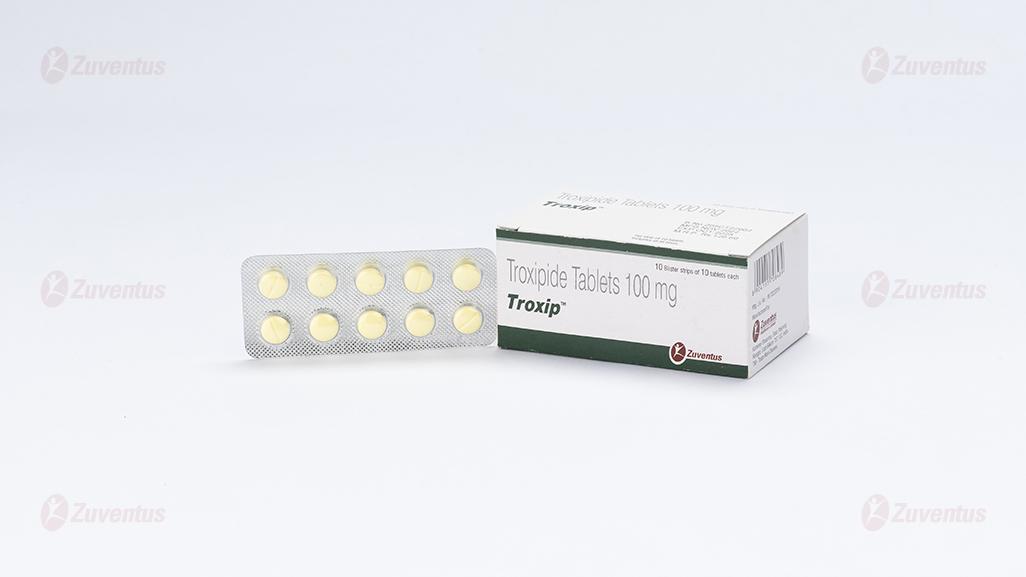
Troxip Tablets
Therapy Area
Gastrointestinal
1.0 Generic name
Troxipide Tablet 100mg
2.0 Qualitative and quantitative composition
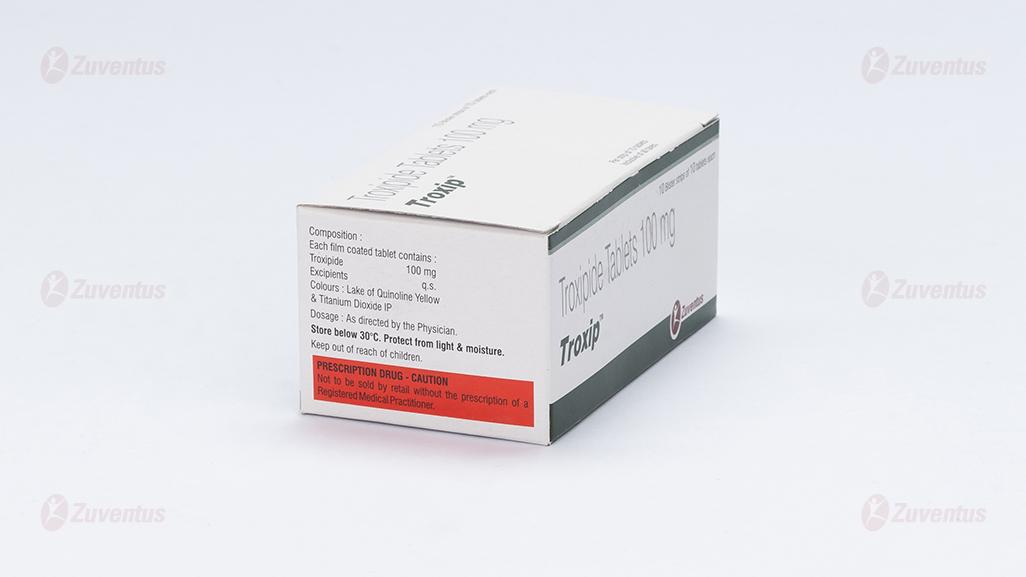
Each film coated tablet contains
Troxipide 100 mg
Colour: Lake quinoline yellow & Titanium Dioxide IP
3.0 Dosage form and strength
Tablet
100mg
4.0 Clinical particulars
4.1 Therapeutic Indication
Troxipide is intended for use in the treatment of acute gastritis, acute exacerbation of chronic gastritis and peptic ulcers
4.2 Posology and method of administration
The recommended dose of Troxipide is 100mg thrice daily for 8-12 weeks
Method of administration: Oral
4.3 Contraindications
Troxipide is contraindicated in patients showing hypersensitivity to Troxipide
4.4 Special warnings and precautions for use
Troxipide should use with caution in children and pregnant women due to lack of safety data. It is known that sexual cycle dysfunction occurred in rats treated with Troxipide. Hence, caution should be exercised while treating women in the reproductive age group. It has to be used with caution in breast feeding women; they should stop breast feeding when on the drug. Troxipide has to be cautiously used in geriatric population.
4.5 Drugs interactions
There have been no reports of interactions with other drugs.
4.6 Use in special populations
Pregnancy and lactation
This drug should be administered to pregnant women or women who may be pregnant only if the therapeutic benefits are deemed to outweigh the risks.
Consider whether to continue or discontinue breast-feeding, taking into account the therapeutic benefits and the benefits of breast-feeding. Transfer of the drug into breast milk has been observed in animal studies (rats).
Children
No clinical trials have been conducted on children or other subjects.
Elderly
In general, physiological functions are often impaired.
4.7 Effects on ability to drive and use machines
Troxib tablets have no influence on the ability to drive and use machines.
4.8 Undesirable effects
Adverse reactions reported with Troxipide are generally mild to moderate in severity and disappear on discontinuation of the drug.
The commonly reported adverse events include:
- Gastrointestinal effects like occasional constipation, abdominal swelling, and stomach discomfort
- Abnormality in liver functions (raised SGOT, SGPT, ALP levels)
- General malaise
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
No cases of overdosage due to Troxip therapy have been reported. In case of accidental overdose, discontinue use and seek professional assistance immediately. Gastric lavage may be necessary to remove drug already released into the stomach.
5.0 Pharmacological properties
5.1 Mechanism of Action
The mechanism of action of this drug is to promote tissue repair of the gastric mucosa by increasing blood flow to the gastric mucosa, normalizing the components of the gastric mucosa, increasing the amount of prostaglandins in the gastric mucosa, and activating the metabolism of the gastric mucosa.
5.2 Pharmacodynamic properties
Troxipide protects against mucosal fragility and disruption of gastric mucosal barrier by increasing the gastric mucosal content of glucosamine and mucopolysaccharides, stimulating the synthesis of cytoprotective prostaglandins and by stimulating the regeneration of collagen fibers. Troxipide is proposed to act by the inhibition of Interleukin 8 (IL-8) stimulated migration of neutrophils in the gastric mucosa. Troxipide also suppresses formyl-methionyl-leucyl-phenylalanine (fMLP) or Platelet Activating Factor (PAF) stimulated superoxide generation and decreases inflammation in the mucosal tissues. It also increases the gastric mucosal blood flow and metabolism
5.3 Pharmacokinetic properties
It is well absorbed throughout the gastrointestinal tract after administration. Troxipide was detected in plasma from 0.05 hr after oral administration of 100 mg of film coated tablets, suggesting a rapid absorption. Bioavailability of Troxipide is 99.40%. A peak serum concentration of 1052.471±51.9318 ng/ml is obtained within 3.042±0. 1896 hrs of drug administration and the resultant area under the curve is 8737.481±315.4253 ng/ml.hr. It is found that, at any time, a mean concentration of 5.3 - 8.9 µg is present per gram of tissue, which is capable of inhibiting the chemotactic migration and superoxide generation in the gastric mucosa. Thus, even 3 hrs after attaining peak serum levels, Troxipide is found in therapeutically active concentrations in the small intestine, liver and stomach. It has a half life of 7.44± 1.85 hrs, and is mainly excreted in the urine (96%) as metabolites [61% after 24 hrs and 87% after 48 hrs].
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
- In a subacute toxicity test in rats, it has been reported that when 1,000 mg/kg/day or more was orally administered, there was more occult blood in the urine, which is thought to be due to inflammation and bleeding in the bladder, than in the control group1)
- Since animal experiments (rats) have reported disturbances of the estrous cycle presumably due to abnormal prolactin secretion2), menstrual abnormalities, lactation, etc. should be closely observed, and appropriate measures such as suspension or discontinuation of the drug should be taken if any abnormalities are found.
7.0 Description
Troxipide, (±)-3,4,-5-trimethoxy-N-3-piperidylbenzamide, is a gastroprotective agent with a molecular formula of C H N O , and molecular weight of 294.3.
The molecular structure of Troxipide is given below.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on pack
8.3 Packaging information
A blister strip of 10 tablets
8.4 Storage and handing instructions
Store in a cool, dry & dark place.
Keep out of reach of children
9.0 Patient Counselling Information
- Troxipide is intended for use in the treatment of acute gastritis, chronic gastritis and peptic ulcers
- Advice patients not to take this medicine, if they are allergic to Troxipide. Signs of an allergic reaction include a rash, itching or shortness of breath.
- Pregnancy: Tell female patients Troxip™ should not be given during pregnancy and lactation. Discuss treatment options with women planning to become pregnant. Tell patients to report pregnancies to their physicians as soon as possible.
- Tell the patients if they get any side effects, talk to the doctor. This includes any possible side effects not listed in this leaflet. Advice patients if they have any further questions, ask the doctor or pharmacist.
- Tablet should be swallowed whole & not to be broken, crushed or chewed. Take it with or after food or a glass of milk.
- Do not stop taking it suddenly without talking to your doctor if you've been on it for a long time.
- Take it with food to avoid an upset stomach.
12.0 Date of revision of text
28/06/2024
About Leaflet
Read all of this leaflet carefully before your child starts taking this medicine because it contains important information for you. –
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for your child. Do not pass it on to others. It may harm them, even if their signs of illness are the same as your child’s.
- If your child gets any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet?
1. What Troxip™ is and what it is used for?
2. What you need to know before you take Troxip™?
3. How to take Troxip™?
4. Possible side effects
5. How to store Troxip™?
6. Contents of the pack and other information
1. What Troxip™ is and what it is used for?
Troxip™ is a tablet composed of Troxipide 100mg.
Troxipide is a mucou-protective, anti-ulcer and anti-inflammatory agent that helps protect your stomach and intestines. It promotes tissue repair by:
- Increasing blood flow to the stomach’s protective layer
- Helping protect the stomach lining from damage.
- Increasing prostaglandins (which are beneficial molecules in the stomach)
- regeneration of the stomach lining and helps restore its normal function.
Troxip™ tablet is used for the treatment of patient with:
- Acute and Chronic Gastritis
- Stomach Ulcers
2. What you need to know before you take Troxip™ Tablet?
Do not take Troxip ™ Tablet:
if you are allergic to Troxipide or any of the ingredients in this medication.
Warnings and Precautions:
- Pregnancy and Breastfeeding: Do not take Troxip™ if you are pregnant or breastfeeding unless your doctor decides it is necessary.
- Children: Safety in children has not been established.
- Elderly: Use with caution due to potential impairment of physiological functions.
Other medicines and Troxip ™ Tablet:
No known drug interaction has been reported yet.
Driving and using machines
Troxib tablets have no influence on the ability to drive and use machines.
3. How to take Troxip ™ Tablet?
Always take Troxip ™ tablet exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure. ]
Dosage in Adults:
The general recommended dosage is one tablet three times a day. The usual treatment duration is 8-12 weeks.
How to Take:
- Swallow the tablet whole with water.
- Take it with or after food or with a glass of milk.
- Do not crush, break, or chew the tablet.
Children and adolescents:
Safety in children has not been established and therefore, Troxip ™ should not be used in this age group
If you take more Troxip ™ tablet than you should
No overdose has been reported; however, it is advisable to go to the nearest casualty department or contact your doctor immediately. Take the tablet carton with you.
If you forget to take Troxip ™ tablet
If you miss a dose, take one as soon as you can. If it is almost time for your next dose, skip the missed dose, do not take two doses at the same time to make up for the missed dose. If you have missed several doses, tell your doctor.
If you stop taking Troxip ™ tablets
Do not stop taking the tablets or reduce the dose without telling your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist
4. Possible side-effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Common side effects include:
- Constipation
- Abdominal swelling
- Stomach discomfort
- Abnormal liver function tests
- General malaise
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top end of the home page.
Website link: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Troxip ™?
Keep this medicine out of the sight and reach of children
Store in a cool, dry & dark place. Protect from light & moisture.
6. Contents of the pack and other information
Pack size: A blister strip of 10 tablets
Each film coated tablet contains
Troxipide 100 mg
Colour: Lake quinoline yellow & Titanium Dioxide IP
Marketing Authorisation Holder
Zuventus Heathcare Ltd.
Zuventus House, Plot Y2, CTS No: 358/A2,
Near Nahur Railway Station,
Nahur (West), Mumbai 400 078.
This leaflet was last revised in June 2024.


![An Open-label, Multicenter Post Marketing Study to Assess the Symptomatic Efficacy and Safety of Troxipide [troxiptm] in the Management of Acid Peptic Disorders in Indian Patients](https://www.zuventus.com/sites/default/files/2023-04/10.-British-Journal-of-Medicine-%26-Medical-Research_9.jpg)