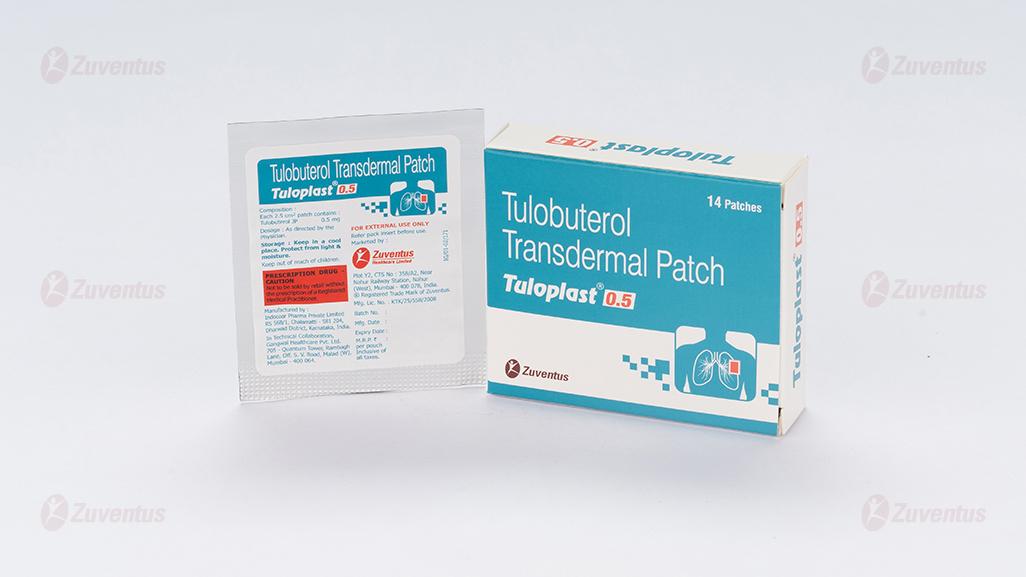
Tuloplast 0.5 mg Patch
Therapy Area
Respiratory
1.0 Generic name
Tulobuterol transdermal patch 0.5 mg / 1 mg/ 2 mg.
2.0 Qualitative and quantitative composition
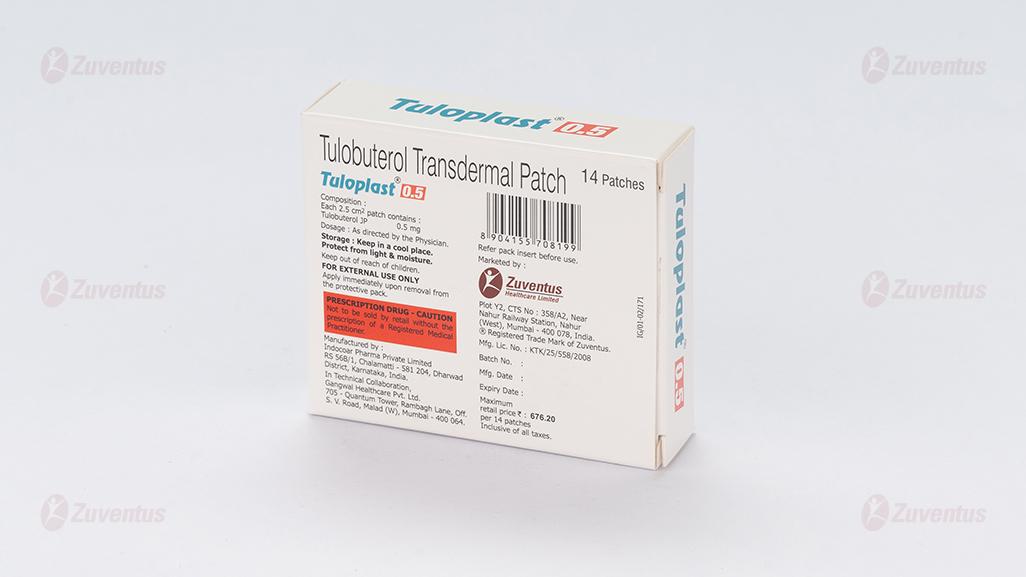
Each 2.5 cm2 patch contains 0.5 mg of tulobuterol
3.0 Dosage form and strength
Transdermal patch
0.5 mg /1 mg /2 mg
4.0 Clinical particulars
4.1 Therapeutic indications
For treatment of patients with asthma and COPD without co-morbidity.
4.2 Posology and method of administration
Posology
Usually, 2 mg for adults, 0.5 mg for children aged between 0.5 and 3 years, 1 mg for children aged between 3 to 9 years or 2 mg aged over 9 years as tulobuterol is applied once daily on the chest, back or upper arm area.
Application site:
1)Before applying tulobuterol, clean and dry the application site.
2)Choose a new site each time to avoid cutaneous irritation.
3)Place tulobuterol on an area that is out of reach of children who may peel it off.
4)Tulobuterol should not be used within the wound as animal studies (rat) showed an increase in the blood level when tulobuterol was applied on the compromised skin.
4.3 Contraindications
Tulobuterol is contraindicated in the following patients: Patients with a history of drug hypersensitivity to any of the ingredients of tulobuterol
4.4 Special warnings and precautions for use
1.Careful Administration (Tulobuterol should be applied with care in the following patients.)
1.Patients with hyperthyroidism [Symptoms may be aggravated.]
2.Patients with hypertension [Blood pressure may be increased.]
3.Patients with heart disorder [Palpitation or arrhythmia may occur.]
4.Patients with diabetes mellitus [Glucose metabolism and blood glucose may increase.]
5.Patients with atopic dermatitis [Pruritus or redness may appear on application site.]
6.Elderly patients
2.Important Precautions
a. Anti-inflammatory drugs such as inhaled steroids are essential for principles of long-term management of the treatment of bronchial asthma. Only if no improvement of symptoms is noted or concomitant treatment with inhaled steroids, etc. are considered appropriate according to the severity of patient's condition, tulobuterol should be applied concomitantly with inhaled steroids. Since tulobuterol is not an alternative anti-inflammatory drug such as inhaled steroids, careful instruction should be given to the patients, the patient's guardian or other appropriate designated person that the patients should not to reduce or discontinue the inhaled steroids, etc. without a physician advice and to use tulobuterol alone even if the patients feel improvement of symptoms with the use of tulobuterol.
b. Careful instruction should be given to the patients, the patient's guardian or other appropriate designated person that the patients should use other appropriate drugs such as short-acting beta-stimulator for acute attack occurred during application of tulobuterol in the long-term management for the treatment of bronchial asthma. Further, if the doses of those drugs are increased or they become ineffective, careful instruction should be given to the patients, the patient's guardian or other appropriate designated person that the patients should visit medical institutions to receive treatment as soon as possible since asthma may not be adequately controlled. As this condition may be life-threatening, intensification of anti-inflammatory therapy should be pursued.
c. If tulobuterol is ineffective even when properly used according to dosage and administration (approx. One to two weeks as a guide), application should be discontinued as tulobuterol is considered inappropriate. In addition, proper instruction and adequate follow-up should be provided for pediatric use.
d. As continue use of tulobuterol beyond the dose range may cause arrhythmia or occasionally cardiac arrest, caution should be given not to use beyond the dose limit.
4.5 Interaction with other medicinal products and other forms of interaction
Precaution for coadminstration (Tulobuterol should be applied with care when coadministered with the following drugs.)
4.6 Use in special populations
Pregnancy & Lactation
- Tulobuterol may be applied to pregnant women or women who may be pregnant only when medical benefits outweigh the risk. [The safety of Tulobuterol in pregnancy has not been established.]
- If Tulobuterol is applied to nursing mothers, breast feeding should be avoided.
Pediatric Use
- The safety of Tulobuterol has not been established in infants less than 6 months of age.
- The safety of long term use of Tulobuterol has not been established in children.
Elderly population
Since physiological function is generally weakened in elderly people, Tulobuterol should be carefully applied, e.g., by starting with lower dose.
4.7 Effects on ability to drive and use machines
No known drug-drug interaction
4.8 Undesirable effect
No investigation of tulobuterol such as drug use surveillance to clarify the incidence of adverse reactions is performed.
1) Clinically significant adverse reactions (incidence unknown) (1) Anaphylactoid symptoms: Since anaphylactoid symptoms may occur, the patient should be closely observed. If any symptoms such as dyspnea, generalized flushing, angioedema and urticaria are noted, application should be discontinued and appropriate measures should be taken.
(2) Serious decreased serum potassium level: Serious decreased serum potassium has been reported with β2 stimulant. Since the serum potassium-lowering effect of β2 stimulant may increase with concomitant use of xanthine derivatives, steroids and diuretics, special caution should be given in patients with severe asthma. Furthermore, hypoxemia may enhance the effect of decreased serum potassium level on cardiac rhythm. In such case, serum potassium level should be monitored.
2) Other adverse reactions
If any symptoms are observed, application of tulobuterol should be discontinued.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
5.0 Pharmacological properties
5.1 Mechanism of action/Pharmacodynamic properties
TULOBUTEROL has a selective β receptor stimulating activity. ATP is converted to cAMP and the contraction of bronchial smooth muscle is suppressed by activating adenylate cyclase after binding to β2 receptor.
5.2 Pharmacokinetic properties
Bioequivalence testing
The concentration of tulobuterol in plasma was measured (crossover method) after single transdermal application (chest, 24 hours) of tulobuterol patch 0.5 mg, 1 mg and 2 mg and standardized preparation corresponding to each standard to healthy adult males, and the statistical analysis of pharmacokinetic parameters (AUC, Cmax) confirmed bioequivalence for both drugs.
(The usual dose in adults is 2 mg per dose as tulobuterol.)
Pharmacokinetic parameters during application of tulobuterol patch
Serum concentration and parameters such as AUC and Cmax may differ from test conditions such as the selection of subjects, number of fluid collected and time.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
No known animal toxicology data
7.0 Description
Tulobuterol is a β2-adrenergic agonist, is the first bronchodilator to be available as a transdermal patch. It is hydrochloride salt of 2-(tert-butylamino)-1-(2-chlorophenyl) ethan-1-ol. Its average molecular weight is 264.191 g/mol
8.0 Patient Counselling Information
- Tuloplast Transdermal Patch is prescribed for the treatment of asthma and COPD.
- Before using Tuloplast Transdermal Patch, clean and dry the application site.
- Apply Tuloplast Transdermal Patch once daily on chest, back, or upper arm as per the specified dosage regimen.
- Do not apply Tuloplast Transdermal Patch to damaged areas (irritated, injured, cut area of skin). Always use a new site for patch application to avoid skin irritation.
- Apply this medicine to a site where children cannot touch, since they might remove the tape.
- Inform your doctor if you are suffering from diabetes, high blood pressure, thyroid disorder, and heart disease.
- Seek medical attention if you experience sudden difficulty in breathing, flushing, swelling of the lips and face, and skin rashes.
- Avoid smoking and minimise your exposure to pollution, dust, pollens and fumes. Along with that, a little exercise each day can help you stay strong.
12.0 Date of revision of the text
14.10.2024
About Leaflet
Please read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
- What Tuloplast is and what it is used for
- What you need to know before you use Tuloplast
- How to use Tuloplast
- Possible side effects
- How to store Tuloplast
- Contents of the pack and other information
1. What Tuloplast is and what it is used for
Tuloplast contains the active substance tulobuterol, which is a bronchodilator. It is used for the treatment of asthma and chronic obstructive pulmonary disease (COPD) without co-morbidity.
2. What you need to know before you use Tuloplast
Do not use Tuloplast:
If you are allergic to tulobuterol or any of the other ingredients of this medicine. Warnings and precautions: Talk to your doctor or pharmacist before using Tuloplast if you have:
- Hyperthyroidism
- Hypertension
- Heart disorders
- Diabetes mellitus
- Atopic dermatitis
Other medicines and Tuloplast:
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines, especially:
- Catecholamine drugs (e.g., adrenaline)
- Xanthine derivatives (e.g., theophylline)
- Steroid drugs (e.g., prednisolone)
- Diuretics (e.g., furosemide)
Pregnancy and breast-feeding:
Tuloplast may be used during pregnancy only if the potential benefit justifies the potential risk. If you are breast-feeding, avoid using Tuloplast as it may pass into breast milk.
Driving and using machines:
Tuloplast has no known effects on the ability to drive or use machines.
IMPORTANT precautions
- Before using Tuloplast Transdermal Patch, clean and dry the application site.
- Apply Tuloplast Transdermal Patch once daily on chest, back, or upper arm as per the specified dosage regimen.
- Do not apply Tuloplast Transdermal Patch to damaged areas (irritated, injured, cut area of skin). Always use a new site for patch application to avoid skin irritation.
- Apply this medicine to a site where children cannot touch, since they might remove the tape.
- Inform your doctor if you are suffering from diabetes, high blood pressure, thyroid disorder, and heart disease.
- Seek medical attention if you experience sudden difficulty in breathing, flushing, swelling of the lips and face, and skin rashes.
- Avoid smoking and minimise your exposure to pollution, dust, pollens and fumes. Along with that, a little exercise each day can help you stay strong.
3. How to take Tuloplast
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Dosage:
- Adults: 2 mg once daily
- Children (0.5 to 3 years): 0.5 mg once daily
- Children (3 to 9 years): 1 mg once daily
- Children (over 9 years): 2 mg once daily
Method of administration:
- Apply the patch to the chest, back, or upper arm.
- Clean and dry the application site before use.
- Use a new site each time to avoid skin irritation.
- Do not apply to damaged skin.
If you apply more Tuloplast® than you should
Please tell your doctor if you experience side effects after incorrect use of this medicine, if you apply more patches than you should. They will be able to advise you of any action that may need to be taken.
If you forget to use Tuloplast®
You should apply a new patch to the affected area when you remember. Do not apply more than one patch to make up for the missed patch.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
- Anaphylactoid symptoms (e.g., dyspnea, generalized flushing, angioedema, urticaria)
- Serious decreased serum potassium levels
Other side effects
- Rash, pruritus, urticaria
- Palpitation, hot flushes, arrhythmia, tachycardia
- Tremor, headache, generalized malaise, sleep loss, dizziness
- Nausea/vomiting, anorexia, stomach discomfort, diarrhea
- Increased AST (GOT), ALT (GPT), eosinophil count
- Application site reactions (e.g., pruritus, redness, rash)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com in and click the tab “Drug Safety Reporting” located on the top end of the home page. Website link: https://www.zuventus.com/drug-safety-reporting. By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store Tuloplast
- Keep out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the outer carton and the sachet after” EXP”. The expiry date refers to the last day of that month.
- Store below 30°C.
- Store in the original package in order to protect from desiccation and light.
- Keep the sachet tightly closed in order to protect from desiccation and light.
- Do not use Tuloplast® if you notice that it is damaged.
- Used patch should be folded in half with the sticky side inwards.
- Do not throw away any medicines via wastewater or household waste. These measures will help protect the environment.
6. Contents of the pack and other information
What Tuloplast contains:
The active substance is tulobuterol.
Each patch contains 0.5 mg, 1 mg, or 2 mg of tulobuterol.
Revised on 09/2024