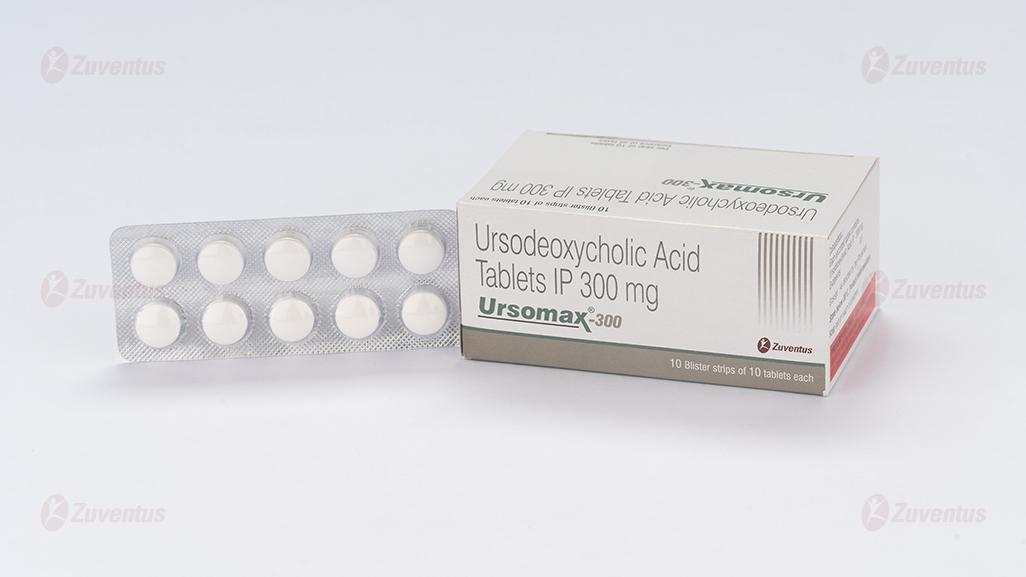
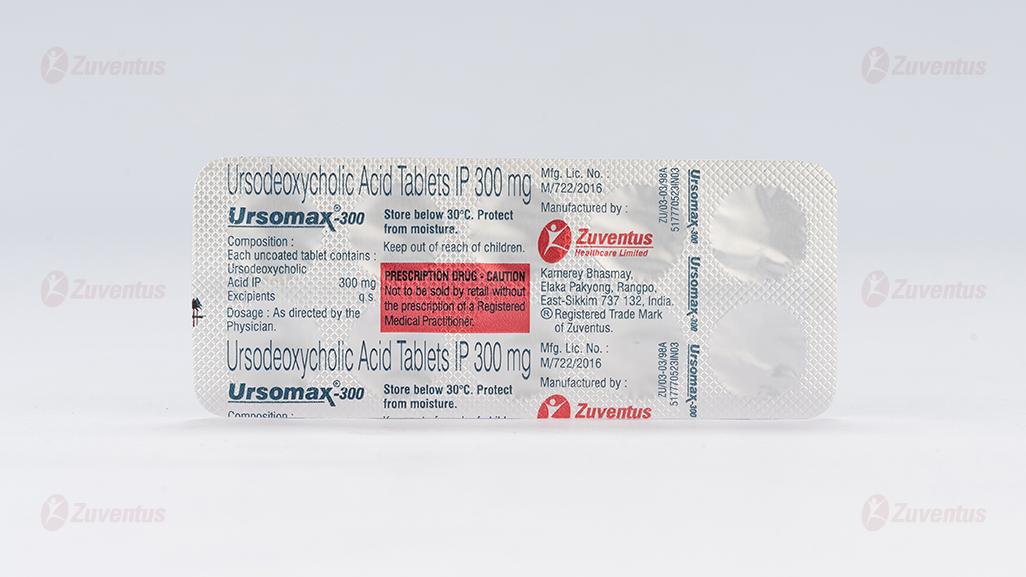
Ursomax 300 Tablets
Therapy Area
Gastrointestinal
1.0 Generic name
Ursodeoxycholic acid
2.0 Qualitative and quantitative composition
Ursomax 150
Each uncoated tablet contains
Ursodeoxycholic acid IP.…………………………150 mg
Excipients……………………………………………...…...q.s.
Ursomax 300
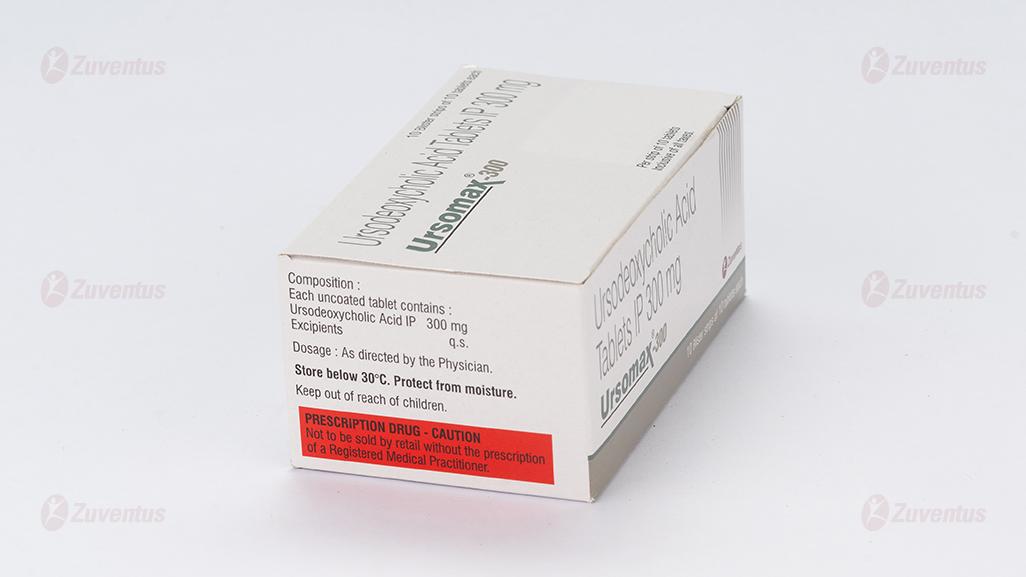
Each uncoated tablet contains
Ursodeoxycholic acid IP.……………………………300 mg
Excipients……………………………………………...…...q.s.
Ursomax 450 SR
Each prolonged-release uncoated tablet contains
Ursodeoxycholic acid IP.……………………………450 mg
Excipients……………………………………………......q.s.
3.0 Dosage form and strength
Tablet 150 mg/300 mg/450 mg
4.0 Clinical particulars
4.1 Therapeutic Indication
For the dissolution of radiolucent cholesterol gallstone, chronic cholestatic liver diseases in particular primary biliary cirrhosis, primary sclerosing cholangitis & cholestasis associated with cystic fibrosis.
4.2 Posology and method of administration
The dosage should be calculated based on the patient's body weight. The calculated dosage should be rounded to the nearest number of tablets.
Dissolving of cholesterol stones
Usual dosage: 8 to 10 mg/kg/day, corresponding to, for example, four to six 150 mg tablets, or two to three tablets of 300 mg, or two tablets of 450 mg. The daily dose can be administered two or three times after the meals: two tablets should always be taken after the evening meal.
Also a single evening dose can be selected (e.g., a patient of 60 kg, in the evening two tablets of 300 mg). Preferably, this single dose should be taken one hour before bedtime and ± two hours after the evening meal with a glass of milk or a small snack.
The duration of the treatment in order to obtain lysis of the gallstones depends on their size, but is usually not shorter than three to four months. To assess the result of the therapy properly, it is necessary to determine the size of the stones accurately at the start of the treatment and to check this further regularly, for example every six months, by means of a new contrast X-ray recording and/or sonographic recording.
In patients in whom, after six months of treatment with the indicated dosage, the stones are not reduced in size, it is recommended to determine the lithogenic index in the bile by means of a duodenum drainage. When the bile has an index of > 1.0, it is unlikely that a favourable result can be obtained, and it is better to consider a different form of treatment for the gallstones.
Treatment should be continued for three to four months after it is established by means of ultrasound check that the gallstones are completely dissolved. An interruption of the treatment for three to four weeks results in a return to over-saturation of the bile and prolongs the overall duration of the therapy. The interruption of the treatment after the dissolving of the gallstones can be followed by a recurrence.
Primary Biliary Cholangitis (Primary biliary cirrhosis)
The dosage of Ursodeoxycholic acid in primary biliary cholangitis (stages I-III), amounts to 12-15 mg/kg/day, which is equivalent to four to eight tablets of 150 mg, two to four tablets of 300 mg, to be taken in two to three portions during the day, or with two tablets of 450 mg, to be taken in two portions during the day.
The dosage of ursodeoxycholic acid in primary biliary cholangitis stage IV and an increase of the serum bilirubin contents (> 40 μg/l), should be in the first instance, only a half of the normal dose (6 to 8 mg/kg/day). Thereafter, the liver function should be closely monitored for several weeks (once every two weeks for six weeks). If there is no deterioration of the liver function (AF, ALT (SGPT), AST (SGOT), γ-GT, bilirubin) and no increase in itching occurs, the dose may be further increased to the usual level. Moreover, the liver function must then again be closely monitored for several weeks. If again no deterioration of the liver function takes place, the patient may be held at the normal dosage for a long time.
In patients with primary biliary cholangitis stage IV without elevated serum bilirubin, the usual starting dose is allowed to be administered directly. Anyway, here too an accurate control of the liver function should be executed.
The treatment of the primary biliary cholangitis should be regularly assessed on the basis of liver values (laboratory) and clinical findings.
Paediatric population
Children with cystic fibrosis aged 6 years to 18 years
Treatment of hepatobiliary diseases as a result of cystic fibrosis 20 mg/kg/day divided in two to three divided doses. If necessary, increase to 30 mg/kg/day. This corresponds with four to ten tablets of 150mg, two to five tablets of 300 mg, or with two to three tablets of 450 mg, to be taken in one or two portions during the day
Method of administration
If the patient has difficulty in swallowing because of the size of the tablet, the tablet can be halved if necessary on the dividing score, so that one half tablet can be taken twice directly in sequence.
4.3 Contraindications
Ursodeoxycholic acid tablets should not be used in patients with:
- Acute inflammation of the gall bladder or bile ducts
- Occlusion of the biliary tract (occlusion of the common bile duct or a cystic duct)
- Frequent episodes of biliary colic
- X-ray radiolucent calcified gallstones
- Impaired contractility of the gallbladder
- Hypersensitivity to bile acids or to any of the excipient
- Active gastric and duodenal ulcers
Paediatric population
- Unsuccessful portoenterostomy or without recovery of good bile flow in children with biliary atresia.
4.4 Special warnings and precautions for use
Ursodeoxycholic acid tablets should be taken under medical supervision.
During the first three months of the treatment liver function parameters AST (SGOT), ALT (SGPT) and γ-GT should be monitored by the physician every 4 weeks, thereafter every 3 months. Apart from allowing for identification of responders and non-responders in patients being treated for primary biliary cholangitis, this monitoring would also enable an early detection of potential hepatic deterioration, particularly in patients with advanced primary biliary cholangitis.
When used for dissolving gallstones:
In order to be able to assess the therapeutic progression of the dissolution of gallstones and to timely identify a possible calcification of the stones, the gall bladder, depending on the size of the stones, should be visualized 6 to 10 months after the start of the treatment (oral cholecystography) with total image and occlusions and in the standing and lying position (ultrasound investigation).
If the gallbladder cannot be visualized on X-rays, or in cases of calcified gallstones, impaired contractility of the gall bladder or frequent episodes of biliary colic, the treatment with Ursodeoxycholic acid should be discontinued.
When used for the treatment of advanced primary biliary cholangitis:
In very rare cases decompensation of liver cirrhosis is observed which partially decreased after treatment discontinuation.
In patients with PBC, the clinical symptoms may worsen in rare cases at the start of treatment, e.g. pruritus may increase. In this case, the therapy is to be continued with a dose reduction and subsequently should be gradually increased to the recommended dose as described in section 4.2.
If diarrhoea occurs, the dosage should be reduced, and treatment should be discontinued in case of persistent diarrhoea.
Female patients who use Ursodeoxycholic acid for dissolving gall stones must use an effective non-hormonal method of contraception, since hormonal contraception may increase biliary lithiasis
4.5 Drugs interactions
- Ursodeoxycholic acid tablets should not be used concurrently with colestyramine, colestipol, or an antacid, on the basis of aluminium hydroxide and/or smectite (aluminium oxide), because these preparations bind ursodeoxycholic acid in the intestine and thereby inhibits its absorption and efficacy. If the use of such a medicine is necessary, must it be taken at least 2 hours before or after ursomax.
- Ursodeoxycholic acid may affect the absorption of ciclosporin from the intestine. In patients treated with ciclosporin the blood level of ciclosporin should be monitored and the ciclosporin dose should be adjusted, if necessary.
- In isolated cases Ursodeoxycholic acid can reduce the absorption of ciprofloxacin.
- In a clinical study in healthy volunteers, the concomitant use of UDCA (500 mg/day) and rosuvastatin (20 mg/day) resulted in slightly elevated plasma levels of rosuvastatin.The clinical relevance of this interaction, also with other statins, is not known.
- Ursodeoxycholic acid has been shown to reduce the peak plasma concentration (Cmax) and the AUC of the calcium antagonist nitrendipine in healthy volunteers. Close monitoring of the outcome of concurrent use of nitrendipine and ursodeoxycholic acid is recommended. An increase of the dose of nitrendipine may be necessary. An interaction with a reduction of the therapeutic effect of dapsone was also reported. These observations, together with in vitro findings could be an indication that ursodeoxycholic acid can induce cytochrome P450 3A enzymes. Induction has, however not been observed in a well-designed interaction study with budesonide, which is a known cytochrome P450 3A substrate.
- Oestrogens and blood cholesterol lowering agents such as clofibrate increase hepatic cholesterol secretion and may therefore encourage biliary lithiasis; which is a counter-effect to ursodeoxycholic acid used for dissolution of gallstones.
4.6 Use in special populations
Pregnancy
- There are no or limited amount of data from the use of ursodeoxycholic acid in pregnant women. Studies in animals have shown reproductive toxicity during the early gestation phase.
- Ursodeoxycholic acid must not be used during pregnancy, unless clearly necessary.
Women of childbearing potentialc
- Women of childbearing potential should be treated with ursodeoxycholic acid, only if they practice reliable contraception: non-hormonal contraceptives or oral contraceptives with low oestrogen dose are recommended. However, in patients taking Ursodeoxycholic acid for dissolving gallstones an effective non-hormonal contraception should be used, since hormonal oral contraceptives may increase biliary lithiasis.
- The possibility of a pregnancy must be excluded before beginning treatment.
Nursing Mothers
- According to few documented cases of breastfeeding women milk levels of ursodeoxycholic acid levels in milk are very low and probably no adverse reactions are to be expected in breastfed infants.
Fertility
Animal studies did not show an influence of ursodeoxycholic acid on fertility. Human data on fertility treatment with ursodeoxycholic acid are not available.
4.7 Effects on ability to drive and use machines
Ursodeoxycholic acid has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
The following adverse reactions have been reported during clinical trials and are ranked using the following frequency:
very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data).
Gastrointestinal disorders:
In clinical studies, reports of pasty stools or diarrhoea during treatment with ursodeoxycholic acid were common.
In very rare cases, severe right upper abdominal pain has occurred during the treatment of primary biliary cholangitis.
Hepatobiliary disorders:
During treatment with ursodeoxycholic acid calcification of gallstones can occur in very rare cases.
During the treatment of advanced stages of primary biliary cholangitis decompensation of cirrhosis has been observed in very rare cases, which partially regressed after treatment discontinuation.
Hypersensitivity reactions:
Very rarely urticaria may occur.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
4.9 Overdose
In the case of overdose diarrhoea may occur. In general, other symptoms of overdose are unlikely, because the absorption of the ursodeoxycholic acid decreases with increasing dose and therefore more is excreted in the faeces.
If diarrhoea occurs, the dosage should be reduced, and treatment should be discontinued in case of persistent diarrhoea. No specific measures are needed and the consequences of diarrhoea should be treated symptomatically with restoration of fluid and electrolyte balance.
Additional information or special populations: Long-term, high-dose UDCA therapy (28-30 mg/kg/day) by patients with primary sclerosing cholangitis (off-label use) was associated with a higher frequency of serious adverse events.
5.0 Pharmacological properties
5.1 Mechanism of Action
The ursodeoxycholic acid converts lithogenic bile in non-lithogenic bile and gradually dissolves the cholesterol gallstones.
5.2 Pharmacodynamic properties
Bile acids are among the most important components of the bile and play a role in the stimulation of bile secretion. Bile acids are also important to keep the cholesterol in bile in solution. In a healthy person, the ratio between the concentration of cholesterol and bile acids in the bile is such that the cholesterol will remain in solution for most of the day. In this case, no gallstones can form (the bile is non-lithogenic). In patients with cholesterol stones in the bile, this ratio is changed and the bile is supersaturated with cholesterol (bile is lithogenic). This may cause a precipitation of cholesterol crystals and the formation of gallstones after some time.
The ursodeoxycholic acid converts lithogenic bile in non-lithogenic bile and gradually dissolves the cholesterol gallstones.
Investigations of the effect of ursodeoxycholic acid on the cholestasis in patients with impaired biliary drainage and on the clinical symptoms in patients with primary biliary cholangitis and cystic fibrosis have shown that cholestatic symptoms in the blood (to be measured by the increased value of alkaline phosphatase (AF), gamma-GT and bilirubin) and the itch declined rapidly, while also the fatigue decreased in the majority of patients. Moreover, studies seem to indicate a positive benefit-risk ratio of the ursodeoxycholic acid in children and young adult cystic fibrosis patients with mild to moderate hepatobiliary disorders.
Paediatric population
Cystic fibrosis
From clinical reports long-term experience of 10 years and more has been gained with UDCA therapy in paediatric patients suffering from cystic fibrosis associated hepatobiliary disorders (CFAHD). There is evidence that treatment with UDCA can inhibit bile duct proliferation, can halt progression of histological damage and even reverse hepato-biliary changes, if it happens at an early stage of CFAHD. The treatment with UDCA should be started as soon as the CFAHD diagnosis is made, in order to optimize the effectiveness of the treatment.
5.3 Pharmacokinetic properties
About 90% of the therapeutic dose of the ursodeoxycholic acid is rapidly absorbed in the small intestine after oral administration.
After the absorption, ursodeoxycholic acid is absorbed in the liver (there is a substantial "first-passeffect"), where it is conjugated with glycine or taurine and then secreted into the bile ducts. Only a small portion of ursodeoxycholic acid is found in the systemic circulation. This is excreted renally. With the exception of conjugation, ursodeoxycholic acid is not metabolised. However, a small fraction of orally administered ursodeoxycholic acid undergoes bacterial conversion to 7-keto-lithocholic acid resp. lithocholic acid after each enterohepatic circulation, while bacterial deconjugation also takes place in the duodenum. Ursodeoxycholic acid, 7-keto-lithocholic acid and lithocholic acid are relatively poorly soluble in water, so a large part of it is excreted via the bile into the faeces. Resorbed ursodeoxycholic acid is conjugated again by the liver; 80% of the lithocholic acid formed in the duodenum is excreted in the faeces, but the remaining 20% of it are sulphated by the liver to insoluble lithocholylconjugates after absorption, which in turn are excreted via the bile and faeces. Resorbed 7- keto-lithocholic acid is reduced to chenodeoxycholic acid in the liver. Lithocholic acid can cause cholestatic liver damage, when the liver is unable to sulphate the lithocholic acid. Although a reduced capacity to sulphate the lithocholic acid in the liver is found in some patients, there is for the time being no clinical evidence that cholestatic liver damage can be associated with the therapy using ursodeoxycholic acid.
After repeated dosage, the ursodeoxycholic acid concentration in the bile reaches a "steady state" after approximately 3 weeks: the total concentration of the ursodeoxycholic acid, however, is never higher than about 60% of the total concentration of the bile acid in the bile: also at high doses.
After therapy with ursodeoxycholic acid is stopped, the concentration of ursodeoxycholic acid in bile decreases quickly after 1 week to 5-10% of the "steady-state" concentration.
The biological half-life of ursodeoxycholic acid is approximately 3.5 to 5.8 days.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Acute toxicity
Acute toxicity studies in animals have not revealed any toxic damage.
b) Chronic toxicity
Subchronic toxicity studies in monkeys showed hepatotoxic effects in the groups given high doses, including functional changes (e.g. liver enzyme changes) and morphological changes such as bile duct proliferation, portal inflammatory foci and hepatocellular necrosis. These toxic effects are most likely attributable to lithocholic acid, a metabolite of ursodeoxycholic acid, which in monkeys – unlike humans – is not detoxified. Clinical experience confirms that the described hepatotoxic effects are of no apparent relevance in humans.
c) Carcinogenic and mutagenic potential
Long-term studies in mice and rats revealed no evidence of ursodeoxycholic acid having carcinogenic potential. In vitro and in vivo genetic toxicology tests with ursodeoxycholic acid were negative. The tests with ursodeoxycholic acid revealed no relevant evidence of a mutagenic effect.
d) Toxicity to reproduction
In studies in rats, tail malformations occurred after a dose of 2000 mg per kg of body weight. In rabbits, no teratogenic effects were found, although there were embryotoxic effects (from a dose of 100 mg per kg of body weight). ursodeoxycholic acid had no effect on fertility in rats and did not affect peri-/post-natal development of the offspring.
7.0 Description
Ursodeoxycholic acid (UDCA) is a naturally occurring bile acid found in small quantities in normal human bile and in larger quantities in the biles of certain species of bears. The chemical name of ursodiol is 3α,7ß-dihydroxy-5ß-cholan-24-oic (C24H40O4)
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on pack
8.3 Packaging information
10 Blister strips of 10 tablets each
8.4 Storage and handing instructions
- Store in a cool, dry & dark place.
- Keep out of reach of children.
- Tablet should be swallowed whole & not to be broken, chewed or crushed.
9.0 Patient counselling information
- Ursomax tablet should be taken after a meal with a glass of milk or water.
- Eat a healthy diet, exercise regularly, and avoid alcohol intake.
- Diarrhea may occur as a side effect. Drink plenty of fluids and inform your doctor if diarrhea persists or if you find blood in your stools.
- Your doctor may monitor your liver function and bilirubin levels every month for the next 3 months after the start of therapy, and every 6 months thereafter.
- Do not stop taking the medication without talking to your doctor.
12.0 Date of Revision
28.08.2024
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What URSOMAX is and what it is used for
2. What you need to know before you take URSOMAX
3. How to take URSOMAX
4. Possible side effects
5. How to store URSOMAX
6. Contents of the pack and other information
1. WHAT URSOMAX IS AND WHAT IT IS USED FOR
The active ingredient in Ursomax tablet is Ursodeoxycholic Acid, which is a naturally occurring substance (bile acid) normally present in the bile.
Ursodeoxycholic Acid influences the composition of the bile, by which cholesterol gallstones can be solved. The effects of Ursodeoxycholic Acid in primary biliary cholangitis and cystic fibrosis can be explained by various mechanisms, such as a protective effect on the liver cells and an effect on the immune system.
1. URSOMAX Tablets is used in patients:
- with small gallstones
- who do not want to undergo surgery or are not eligible for gallstone surgery
- in whom too much cholesterol has been found in the bile
2. URSOMAX Tablets is used in patients with primary biliary cholangitis. Primary biliary cholangitis is a disease in which liver tissue is damaged by an impaired drainage of the bile.
3. URSOMAX Tablets is used in children aged 6 years to 18 years old with liver and biliary diseases caused by cystic fibrosis. Cystic fibrosis, also referred to as mucoviscidosis, is an inherited disorder in which the mucus is particularly tough in the whole body. This may cause, among other conditions, clogging and inflammation in the liver and in the bile ducts.
2. WHAT YOU NEED TO KNOW BEFORE YOU TAKE URSOMAX
Do not use URSOMAX
- You have an acute inflammation of the gallbladder or the bile ducts.
- You have a narrowing or blockage of the bile ducts.
- You suffer from often occurring cramp-like pain in the upper abdomen (biliary colic).
- You have calcified gallstones that do not transmit X-rays.
- You have a gall bladder that cannot properly constrict any more.
- You are allergic to bile acids or any of the other ingredients of this medicine.
- You have an active gastric or duodenal ulcer.
Children
In children with disrupted biliary drainage due to production of connective tissue in the bile duct (biliary atresia) in whom the bile flow is not restored by healing or by an artificial bile duct (portoenterostomy).
Warnings and precautions
This medicine must be used under medical supervision.
Your doctor should examine your liver every 4 weeks during the first three months of treatment. Then this should be done every 3 months. Apart from allowing for identification of responders and non-responders in patients being treated for primary biliary cholangitis, this monitoring would also enable an early detection of potential hepatic deterioration, particularly in patients with advanced primary biliary cholangitis.
When used for dissolving gallstones:
In order to be able to assess the therapeutic progression of the dissolution of gallstones and to timely identify a possible calcification of the stones, the gall bladder, depending on the size of the stones, should be visualized 6 to 10 months after the start of the treatment (oral cholecystography) with total image and occlusions and in the standing and lying position (ultrasound control).
If the gallbladder cannot be visualized on X-rays, or in cases of calcified gallstones, impaired contractility of the gall bladder or frequent episodes of biliary colic, the treatment with Ursodeoxycholic Tablets should be discontinued.
When used for the treatment of advanced primary biliary cholangitis:
In very rare cases decompensation of liver cirrhosis is observed which partially decreased after treatment discontinuation. Women who use URSOMAX for dissolving gallstones should stop using the birth control pill and other methods used to prevent pregnancy, because the hormones in the birth control pill can promote the production of gallstones.
When you are in the final stage of primary biliary cholangitis it can in very rare cases occur that your liver function is strongly reduced. The liver function will partly recover after stopping the treatment.
If you experience problems with diarrhoea, your doctor will reduce the dose. If the diarrhoea persists, your doctor may decide to stop the treatment.
Contact your doctor or pharmacist before you start to use this medicine.
Other medicines and URSOMAX Tablets
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This also applies for medicines obtained without a prescription.
The effect of the following drugs can be influenced (interactions):
A reduced effect of the following medicines is possible when using this medicine:
- Medicines binding stomach acid based on aluminium hydroxide and bile acid binding substances (colestyramine, colestipol) can bind the ursodeoxycholic acid and thereby prevent its absorption. Therefore, these medicines may not be taken simultaneously with URSOMAX, but always 2 hours before or after.
- Ursodeoxycholic acid can reduce the absorption of ciprofloxacin, dapsone (antibiotics) and nitrendipine (antihypertensive agent) from the intestine. When one of these resources has been used simultaneously with URSOMAX, your doctor will carefully supervise you.
An intensified effect of the following medicines is possible when using this medicine:
- URSOMAX may increase the absorption of cyclosporine from the intestine: if necessary, the dosing guided by the cyclosporine concentration should be adjusted in the blood.
Oestrogens, oral contraceptives ("the pill") and cholesterol-lowering agents (such as clofibrate) may promote the forming of gallstones, and can thus counteract the effect of the treatment of gallstones with URSOMAX Tablets
Inform your doctor or pharmacist, if you are taking or have recently taken other medicines. This also applies for medicines obtained without a prescription.
Pregnancy and breast-feeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy:
You should not use this medicine during pregnancy unless your doctor considers it absolutely necessary.
Women of child-bearing potential
Even if you are not pregnant, you should discuss this possibility with your doctor, because women of childbearing age may only be treated, if they use a reliable method of contraception. Non-hormonal contraception or contraception with low dose oestrogens is recommended. However, if you use this medicine for solving gallstones, you may only use non-hormonal contraception, because hormonal contraception promotes the formation of gallstones.
Breast-feeding
Tell your doctor if you are breastfeeding or about to start breastfeeding. According to few documented cases of breastfeeding women milk levels of ursodeoxycholic acid in milk are very low and probably no adverse reactions are to be expected in breastfed infants.
Driving and using machines
URSOMAX has no or negligible influence on driving ability or the ability to operate machines.
3. HOW TO USE URSOMAX
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Your doctor will determine your dose based on your body weight.
Take the tablets after a meal with a glass of milk or a small snack. Take the prescribed number of tablets distributed throughout the day.
1. Dissolving of gallstones:
Four to six tablets of 150 mg, two to three tablets of 300 mg or two tablets of 450 mg per day (600- 900 mg ursodeoxycholic acid per day).
Two tablets should always be taken after dinner.
When taking a dose two or three times daily: for example, one tablet after lunch and two tablets after dinner.
When taking one dose of two tablets daily: take both tablets preferably two hours after dinner one hour before going to sleep.
2. Primary biliary cholangitis (damage to liver tissue by impaired bile flow):
- Phase I-III: four to eight tablets of 150 mg, two to four tablets of 300 mg, or two tablets of 450 mg per day (600-1200 mg ursodeoxycholic acid per day).
Take the prescribed dose in two to three servings per day after meals.
- Phase IV: On the basis of liver function examination your doctor will determine if a normal dosage, as in phase I-III, or the half of this dose will be prescribed to you.
Children with cystic fibrosis aged 6 to 18 years
3. Disorders of liver and biliary tract caused by cystic fibrosis (mucoviscidosis):
four to ten tablets of 150 mg, two to five tablets of 300 mg, or two to three tablets of 450 mg per day (600-1500 mg ursodeoxycholic acid per day).
The tablets with a score can be divided if you have problems in swallowing because of the size of the tablets, so that one half tablet can be taken twice directly in sequence.
If you notice that Ursodeoxycholic Acid Tablets is too strong or on the contrary too weak, consult your doctor or pharmacist.
If you take more URSOMAX than you should
You should tell your doctor if you have taken more Ursodeoxycholic Acid Tablets than you should. It is unlikely that you will notice any problems but you may experience diarrhoea.
If you forget to take URSOMAX
Take the prescribed amount at the next regular taking time. Do not take a double dose to make up for a forgotten dose.
How long should you use URSOMAX Tablets?
The duration of the treatment depends on the size of the gallstone, but is usually not shorter than three to four months. Treatment should not be interrupted prematurely; even if the symptoms have disappeared.
Only an X-ray or an ultrasound scan can show that the gallstones are completely dissolved. After it has been shown with the aid of an echogram that the gallstones have disappeared completely, the treatment should still be continued for three to four months.
The use of Ursodeoxycholic Acid in the treatment of primary biliary cholangitis and disorders of the liver and the biliary system as a result of cystic fibrosis will usually be maintained continuously, in order to continue to maintain the protective effect of Ursodeoxycholic Acid.
Do you have any further questions on the use of this medicine? Please contact your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects (occurring in fewer than 1 in 10 patients but more than 1 in 100 patients)
- pasty stools or diarrhoea
Very rare side effects (may affect up to 1 in 10,000 people)
- in the treatment of primary biliary cholangitis: severe pain in the right upper abdomen, severe deterioration (decompensation) of the liver cirrhosis which partially decreases after cessation of treatment;
- calcification of gallstones;
- urticaria (hives)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. HOW TO STORE URSOMAX
- Store in a cool, dry & dark place.
- Keep out of reach of children.
- Tablet should be swallowed whole & not to be broken, chewed or crushed.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What URSOMAX contains
Ursomax 150
Each uncoated tablet contains
Ursodeoxycholic acid IP.……………………………150 mg
Excipients……………………………………………..…...q.s.
Ursomax 300
Each uncoated tablet contains
Ursodeoxycholic acid IP.……………………………300 mg
Excipients……………………………………………..…...q.s.
Ursomax 450 SR
Each prolonged-release uncoated tablet contains
Ursodeoxycholic acid IP.……………………………450 mg
Excipients……………………………………………..…...q.s.
What URSOMAX looks like and contents of the pack
10 Blister strips of 10 tablets each
Marketing authorisation holder and manufacturer
Zuventus Healthcare Ltd
Kamerey Bhasmay, Elaka Pakyong,
Rangpo, East-Sikkim 737 132.
Registered Trade Mark of Zuventus.
This leaflet was last revised in 08/2023