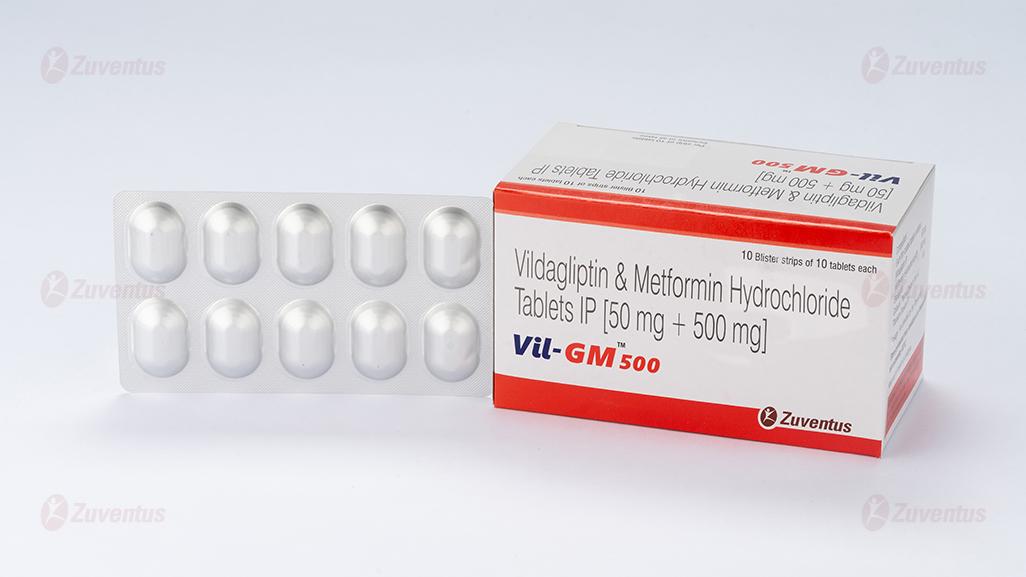
Vil GM 500 Tablets
Therapy Area
Anti-diabetic
1.0 Generic name
Vildagliptin & Metformin Hydrochloride Tablets IP
2.0 Qualitative and quantitative composition
Vil GM 500
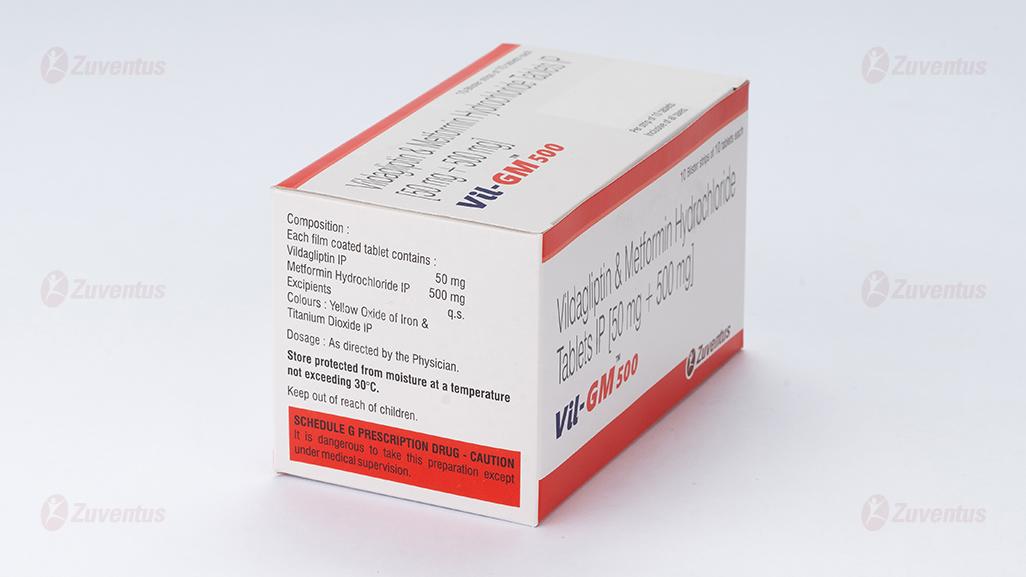
Each film coated tablet contains :
Vildagliptin IP 50 mg
Metformin Hydrochloride IP 500 mg
Excipients q.s.
Colours : Yellow Oxide of Iron & Titanium Dioxide IP
Vil GM 1000
Each film coated tablet contains :
Vildagliptin IP 50 mg
Metformin Hydrochloride IP 1000 mg
Excipients
Colours : Yellow Oxide of Iron & Titanium Dioxide IP
3.0 Dosage form and strength
Tablet, 50 mg / 500 mg and 50 mg / 1000 mg
4.0 Clinical particulars
4.1 Therapeutic indication
For the treatment of type-II diabetes mellitus when single drug therapy along with diet, exercise do not result in adequate glycemic control.
4.2 Posology and method of administration
Adults with normal renal function (GFR ≥ 90 ml/min) The dose of antihyperglycaemic therapy with Vil GM should be individualised on the basis of the patient's current regimen, effectiveness and tolerability while not exceeding the maximum recommended daily dose of 100 mg Vildagliptin.
Vil GM may be initiated at either the 50 mg / 500 mg or 50 mg / 1000 mg tablet strength twice daily, one tablet in the morning and the other in the evening. For patients inadequately controlled at their maximal tolerated dose of Metformin monotherapy :
The starting dose of Vil GM should provide Vildagliptin as 50 mg twice daily (100 mg total daily dose) plus the dose of Metformin already being taken. For patients switching from co-administration of Vildagliptin and Metformin as separate tablets :
Vil GM should be initiated at the dose of Vildagliptin and Metformin already being taken. For patients inadequately controlled on dual combination with Metformin and a Sulphonylurea :
The doses of Vil GM should provide Vildagliptin as 50 mg twice daily (100 mg total daily dose) and a dose of Metformin similar to the dose already being taken. When Vil GM is used in combination with a Sulphonylurea, a lower dose of the Sulphonylurea may be considered to reduce the risk of hypoglycaemia. For patients inadequately controlled on dual combination therapy with insulin and the maximal tolerated dose of Metformin :
The dose of Vil GM should provide Vildagliptin dosed as 50 mg twice daily (100 mg total daily dose) and a dose of Metformin similar to the dose already being taken. The safety and efficacy of Vildagliptin and Metformin as triple oral therapy in combination with a Thiazolidinedione have not been established.
Special populations
Elderly (≥ 65 years)
As Metformin is excreted via the kidney, and elderly patients have a tendency to decreased renal function, elderly patients taking Vil GM should have their renal function monitored regularly.
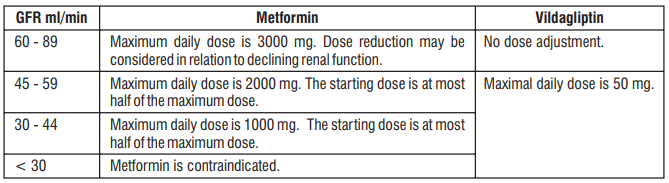
Renal impairment
A GFR should be assessed before initiation of treatment with Metformin-containing products and at least annually thereafter. In patients at increased risk of further progression of renal impairment and in the elderly, renal function should be assessed more frequently, e.g. every 3 - 6 months. The maximum daily dose of Metformin should preferably be divided into 2 - 3 daily doses. Factors that may increase the risk of lactic acidosis should be reviewed before considering initiation of Metformin in patients with GFR < 60 ml/min.
If no adequate strength of Vil GM is available, individual monocomponents should be used instead of the fixed dose combination.
Hepatic impairment
Vil GM should not be used in patients with hepatic impairment, including those with pre-treatment alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 3 times the upper limit of normal (ULN).
Paediatric population
Vil GM is not recommended for use in children and adolescents (< 18 years). The safety and efficacy of Vil GM in children and adolescents (< 18 years) have not been established. No data are available.
Method of administration
Oral use
Taking Vil GM with or just after food may reduce gastrointestinal symptoms associated with Metformin
4.3 Contraindications
- Hypersensitivity to the active substances or to any of the excipients.
- Any type of acute metabolic acidosis. (such as lactic acidosis, diabetic ketoacidosis.)
- Diabetic pre-coma
- Severe renal failure (GFR < 30 ml/min)
- Acute conditions with the potential to alter renal function, such as
- Dehydration
- Severe infection
- Shock
- Intravascular administration of iodinated contrast agents
- Acute or chronic disease which may cause tissue hypoxia, such as
- Cardiac or respiratory failure
- Recent myocardial infarction
- Shock
- Hepatic impairment
- Acute alcohol intoxication, alcoholism
- Breast-feeding
4.4 Special warnings and precautions for use
General
Vil GM is not a substitute for insulin in insulin-requiring patients and should not be used in patients with type 1 diabetes
Lactic acidosis
Lactic acidosis, a very rare but serious metabolic complication, most often occurs at acute worsening of renal function, or cardiorespiratory illness or sepsis. Metformin accumulation occurs at acute worsening of renal function and increases the risk of lactic acidosis.
In case of dehydration (severe diarrhoea or vomiting, fever or reduced fluid intake), Metformin should be temporarily discontinued and contact with a health care professional is recommended.
Medicinal products that can acutely impair renal function (such as antihypertensives, diuretics and NSAIDs) should be initiated with caution in Metformin-treated patients. Other risk factors for lactic acidosis are excessive Alcohol intake, hepatic insufficiency, inadequately controlled diabetes, ketosis, prolonged fasting and any conditions associated with hypoxia, as well as concomitant use of medicinal products that may cause lactic acidosis. Patients and/or care-givers should be informed of the risk of lactic acidosis. Lactic acidosis is characterised by acidotic dyspnoea, abdominal pain, muscle cramps, asthenia and hypothermia followed by coma. In case of suspected symptoms, the patient should stop taking Metformin and seek immediate medical attention. Diagnostic laboratory findings are decreased blood pH (< 7.35), increased plasma lactate levels (> 5 mmol/l) and an increased anion gap and lactate/pyruvate ratio.
Administration of iodinated contrast agents
Intravascular administration of iodinated contrast agents may lead to contrast-induced nephropathy, resulting in Metformin accumulation and increased risk of lactic acidosis. Metformin should be discontinued prior to or at the time of the imaging procedure and not restarted until at least 48 hours after, provided that renal function has been reevaluated and found to be stable
Renal function
GFR should be assessed before treatment initiation and regularly thereafter. Metformin is contraindicated in patients with GFR < 30 ml/min and should be temporarily discontinued in the presence of conditions that alter renal function.
Hepatic impairment
Patients with hepatic impairment, including those with pre-treatment ALT or AST > 3x ULN, should not be treated with Vil GM
Liver enzyme monitoring
Rare cases of hepatic dysfunction (including hepatitis) have been reported with Vildagliptin. In these cases, the patients were generally asymptomatic without clinical sequelae and liver function tests (LFTs) returned to normal after discontinuation of treatment. LFTs should be performed prior to the initiation of treatment with Vil GM in order to know the patient's baseline value. Liver function should be monitored during treatment with Vil GM at three-month intervals during the first year and periodically thereafter. Patients who develop increased transaminase levels should be monitored with a second liver function evaluation to confirm the finding and be followed thereafter with frequent LFTs until the abnormality(ies) return(s) to normal. Should an increase in AST or in ALT of 3x ULN or greater persist, withdrawal of Vil GM therapy is recommended. Patients who develop jaundice or other signs suggestive of liver dysfunction should discontinue Vil GM. Following withdrawal of treatment with Vil GM and LFT normalisation, treatment with Vil GM should not be re-initiated.
Skin disorders
Skin lesions, including blistering and ulceration have been reported with Vildagliptin in extremities of monkeys in nonclinical toxicology studies. Although skin lesions were not observed at an increased incidence in clinical trials, there was limited experience in patients with diabetic skin complications. Furthermore, there have been post-marketing reports of bullous and exfoliative skin lesions. Therefore, in keeping with routine care of the diabetic patient, monitoring for skin disorders, such as blistering or ulceration, is recommended.
Acute pancreatitis
Use of Vildagliptin has been associated with a risk of developing acute pancreatitis. Patients should be informed of the characteristic symptom of acute pancreatitis. If pancreatitis is suspected, Vildagliptin should be discontinued; if acute pancreatitis is confirmed, Vildagliptin should not be restarted. Caution should be exercised in patients with a history of acute pancreatitis.
Hypoglycaemia
Sulphonylureas are known to cause hypoglycaemia. Patients receiving Vildagliptin in combination with a Sulphonylurea may be at risk for hypoglycaemia. Therefore, a lower dose of Sulphonylurea may be considered to reduce the risk of hypoglycaemia.
Surgery
Metformin must be discontinued at the time of surgery under general, spinal or epidural anaesthesia. Therapy may be restarted no earlier than 48 hours following surgery or resumption of oral nutrition and provided that renal function has been re-evaluated and found to be stable.
4.5 Drugs interactions
There have been no formal interaction studies for Vil GM. The following statements reflect the information available on the individual active substances.
Vildagliptin
- Vildagliptin has a low potential for interactions with co-administered medicinal products. Since Vildagliptin is not a cytochrome P (CYP) 450 enzyme substrate and does not inhibit or induce CYP 450 enzymes, it is not likely to interact with active substances that are substrates, inhibitors or inducers of these enzymes.
- Results from clinical trials conducted with the oral antidiabetics Pioglitazone, Metformin and Glyburide in combination with Vildagliptin have shown no clinically relevant pharmacokinetic interactions in the target population.
- Drug-drug interaction studies with Digoxin (P-glycoprotein substrate) and Warfarin (CYP2C9 substrate) in healthy subjects have shown no clinically relevant pharmacokinetic interactions after co-administration with Vildagliptin.
- Drug-drug interaction studies in healthy subjects were conducted with Amlodipine, Ramipril, Valsartan and Simvastatin. In these studies, no clinically relevant pharmacokinetic interactions were observed after coadministration with Vildagliptin. However, this has not been established in the target population.
- Combination with ACE inhibitors.
- There may be an increased risk of angioedema in patients concomitantly taking ACE inhibitors.
- As with other oral antidiabetic medicinal products the hypoglycaemic effect of Vildagliptin may be reduced by certain active substances, including Thiazides, Corticosteroids, Thyroid products and Sympathomimetics.
Metformin
Combinations not recommended
- Alcohol Alcohol intoxication is associated with an increased risk of lactic acidosis, particularly in cases of fasting, malnutrition or hepatic impairment.
- Iodinated contrast agents Metformin must be discontinued prior to or at the time of the imaging procedure and not restarted until at least 48 hours after, provided that renal function has been re-evaluated and found to be stable.
- Cationic active substances Cationic active substances that are eliminated by renal tubular secretion (e.g. Cimetidine) may interact with Metformin by competing for common renal tubular transport systems and hence delay the elimination of Metformin, which may increase the risk of lactic acidosis. A study in healthy volunteers showed that Cimetidine, administered as 400 mg twice daily, increased Metformin systemic exposure (AUC) by 50%. Therefore, close monitoring of glycaemic control, dose adjustment within the recommended posology and changes in diabetic treatment should be considered when cationic medicinal products that are eliminated by renal tubular secretion are co-administered.
Combinations requiring precautions for use
- Some medicinal products can adversely affect renal function which may increase the risk of lactic acidosis, e.g. NSAIDs, including selective Cyclo-oxygenase (COX) II inhibitors, ACE inhibitors, angiotensin II receptor antagonists and diuretics, especially loop diuretics. When starting or using such products in combination with Metformin, close monitoring of renal function is necessary.
- Glucocorticoids, beta-2-agonists and diuretics have intrinsic hyperglycaemic activity. The patient should be informed and more frequent blood glucose monitoring performed, especially at the beginning of treatment. If necessary, the dosage of Vil GM may need to be adjusted during concomitant therapy and on its discontinuation.
- Angiotensin converting enzyme (ACE) inhibitors may decrease the blood glucose levels. If necessary, the dosage of the antihyperglycaemic medicinal product should be adjusted during therapy with the other medicinal product and on its discontinuation.
4.6 Use in special populations
Pregnancy
There are no adequate data from the use of Vil GM in pregnant women. For Vildagliptin studies in animals have shown reproductive toxicity at high doses. For Metformin, studies in animals have not shown reproductive toxicity. Studies in animals performed with Vildagliptin and Metformin have not shown evidence of teratogenicity, but foetotoxic effects at maternotoxic doses. The potential risk for humans is unknown. Vil GM should not be used during pregnancy
Nursing mothers Studies in animals have shown excretion of both Metformin and Vildagliptin in milk. It is unknown whether Vildagliptin is excreted in human milk, but Metformin is excreted in human milk in low amounts. Due to both the potential risk of neonate hypoglycaemia related to Metformin and the lack of human data with Vildagliptin, Vil GM should not be used during breast-feeding
Fertility No studies on the effect on human fertility have been conducted for Vil GM.
4.7 Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. Patients who may experience dizziness as an adverse reaction should avoid driving vehicles or using machines.
4.8 Undesirable effects
Arthralgia is reported with Vildagliptin in recent reports. Adverse reactions reported in patients who received Vildagliptin and Metformin in double-blind studies are listed below by system organ class and absolute frequency. Frequencies are defined as very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥ 1/10,000 to < 1/1,000), very rare (< 1/10,000), not known (cannot be estimated from the available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
- Metabolism and nutrition disorders
Common - Hypoglycaemia
- Nervous system disorders
Common - Tremor, Headache, Dizziness
Uncommon - Fatigue
- Gastrointestinal disorders
Common - Nausea
In controlled clinical trials with the combination of Vildagliptin 100 mg daily plus Metformin, no withdrawal due to adverse reactions was reported in either the Vildagliptin 100 mg daily plus Metformin or the placebo plus Metformin treatment groups. Clinical trials of up to more than 2 years' duration did not show any additional safety signals or unforeseen risks when Vildagliptin was added on to Metformin.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com Website : https://www.zuventus.com/drug-safety-reporting
4.9 Overdose
Symptoms
Vildagliptin
Information regarding overdose with Vildagliptin is limited. Information on the likely symptoms of overdose was taken from a rising dose tolerability study in healthy subjects given Vildagliptin for 10 days. At 400 mg, there were three cases of muscle pain, and individual cases of mild and transient paraesthesia, fever, oedema and a transient increase in lipase levels. At 600 mg, one subject experienced oedema of the feet and hands, and increases in creatine phosphokinase (CPK), aspartate aminotransferase (AST), Creactive protein (CRP) and myoglobin levels. Three other subjects experienced oedema of the feet, with paraesthesia in two cases. All symptoms and laboratory abnormalities resolved without treatment after discontinuation of the study medicinal product.
Metformin
A large overdose of Metformin (or co-existing risk of lactic acidosis) may lead to lactic acidosis, which is a medical emergency and must be treated in hospital.
Management
The most effective method of removing Metformin is haemodialysis. However, Vildagliptin cannot be removed by haemodialysis, although the major hydrolysis metabolite (LAY 151) can. Supportive management is recommended.
5.0 Pharmacological properties
5.1 Mechanism of action
Vil GM combines two antihyperglycaemic agents with complimentary mechanisms of action to improve glycaemic control in patients with type 2 diabetes : Vildagliptin, a member of the islet enhancer class, and Metformin Hydrochloride, a member of the biguanide class. The administration of Vildagliptin results in a rapid and complete inhibition of DPP-4 activity, resulting in increased fasting and postprandial endogenous levels of the incretin hormones GLP-1 (Glucagon-Like Peptide 1) and GIP (Glucose-dependent Insulinotropic Polypeptide). Metformin acts primarily by decreasing endogenous hepatic glucose production.
5.2 Pharmacodynamic properties
Vildagliptin
By increasing the endogenous levels of these incretin hormones, Vildagliptin enhances the sensitivity of beta cells to glucose, resulting in improved glucose-dependent insulin secretion. Treatment with Vildagliptin 50 - 100 mg daily in patients with type 2 diabetes significantly improved markers of beta cell function including HOMA-β (Homeostasis Model Assessment-β), proinsulin to insulin ratio and measures of beta cell responsiveness from the frequentlysampled meal tolerance test. In non-diabetic (normal glycaemic) individuals, Vildagliptin does not stimulate insulin secretion or reduce glucose levels.
By increasing endogenous GLP-1 levels, Vildagliptin also enhances the sensitivity of alpha cells to glucose, resulting in more glucose-appropriate glucagon secretion. The enhanced increase in the insulin/glucagon ratio during hyperglycaemia due to increased incretin hormone levels results in a decrease in fasting and postprandial hepatic glucose production, leading to reduced glycaemia. The known effect of increased GLP-1 levels delaying gastric emptying is not observed with Vildagliptin treatment.
Metformin
Metformin is a biguanide with antihyperglycaemic effects, lowering both basal and postprandial plasma glucose. It does not stimulate insulin secretion and therefore does not produce hypoglycaemia or increased weight gain. Metformin may exert its glucose-lowering effect via three mechanisms :
- By reduction of hepatic glucose production through inhibition of gluconeogenesis and glycogenolysis.
- In muscle, by modestly increasing insulin sensitivity, improving peripheral glucose uptake and utilisation.
- By delaying intestinal glucose absorption.
Metformin stimulates intracellular glycogen synthesis by acting on glycogen synthase and increases the transport capacity of specific types of membrane glucose transporters (GLUT-1 and GLUT-4). In humans, independently of its action on Glycaemia, Metformin has favourable effects on lipid metabolism.
5.3 Pharmacokinetic properties
Vil GM
Bioequivalence has been demonstrated between Vil GM at three dose strengths (50 mg / 500 mg, 50 mg / 850 mg and 50 mg / 1000 mg) versus free combination of Vildagliptin and Metformin Hydrochloride tablets at the corresponding doses. Food does not affect the extent and rate of absorption of Vildagliptin from Vil GM. The rate and extent of absorption of Metformin from Vil GM 50 mg / 1000 mg were decreased when given with food as reflected by the decrease in Cmax by 26%, AUC by 7% and delayed Tmax (2.0 to 4.0 h). The following statements reflect the pharmacokinetic properties of the individual active substances of Vil GM.
Vildagliptin
Absorption
Following oral administration in the fasting state, Vildagliptin is rapidly absorbed, with peak plasma concentrations observed at 1.7 hours. Food slightly delays the time to peak plasma concentration to 2.5 hours, but does not alter the overall exposure (AUC). Administration of Vildagliptin with food resulted in a decreased Cmax (19%). However, the magnitude of change is not clinically significant, so that Vildagliptin can be given with or without food. The absolute bioavailability is 85%.
Distribution
The plasma protein binding of Vildagliptin is low (9.3%) and Vildagliptin distributes equally between plasma and red blood cells. The mean volume of distribution of Vildagliptin at steady-state after intravenous administration (Vss) is 71 litres, suggesting extravascular distribution.
Biotransformation
Metabolism is the major elimination pathway for Vildagliptin in humans, accounting for 69% of the dose. The major metabolite (LAY 151) is pharmacologically inactive and is the hydrolysis product of the cyano moiety, accounting for 57% of the dose, followed by the glucuronide (BQS867) and the amide hydrolysis products (4% of dose). In vitro data in human kidney microsomes suggest that the kidney may be one of the major organs contributing to the hydrolysis of Vildagliptin to its major inactive metabolite, LAY151. DPP-4 contributes partially to the hydrolysis of Vildagliptin based on an in vivo study using DPP-4 deficient rats. Vildagliptin is not metabolised by CYP 450 enzymes to any quantifiable extent. Accordingly, the metabolic clearance of Vildagliptin is not anticipated to be affected by co-medications that are CYP 450 inhibitors and/or inducers. In vitro studies demonstrated that Vildagliptin does not inhibit/induce CYP 450 enzymes. Therefore, Vildagliptin is not likely to affect metabolic clearance of co-medications metabolised by CYP 1A2, CYP 2C8, CYP 2C9, CYP 2C19, CYP 2D6, CYP 2E1 or CYP 3A4/5.
Elimination
Following oral administration of [14C] Vildagliptin, approximately 85% of the dose was excreted into the urine and 15% of the dose is recovered in the faeces. Renal excretion of the unchanged Vildagliptin accounted for 23% of the dose after oral administration. After intravenous administration to healthy subjects, the total plasma and renal clearances of Vildagliptin are 41 and 13 l/h, respectively. The mean elimination half-life after intravenous administration is approximately 2 hours. The elimination half-life after oral administration is approximately 3 hours.
Metformin
Absorption
After an oral dose of Metformin, the maximum plasma concentration (Cmax) is achieved after about 2.5 h. Absolute bioavailability of a 500 mg Metformin tablet is approximately 50 - 60% in healthy subjects. After an oral dose, the nonabsorbed fraction recovered in faeces was 20 - 30%. After oral administration, Metformin absorption is saturable and incomplete. It is assumed that the pharmacokinetics of Metformin absorption are non-linear. At the usual Metformin doses and dosing schedules, steady state plasma concentrations are reached within 24 - 48 h and are generally less than 1 µg/ml. In controlled clinical trials, maximum Metformin plasma levels (Cmax) did not exceed 4 µg/ml, even at maximum doses. Food slightly delays and decreases the extent of the absorption of Metformin. Following administration of a dose of 850 mg, the plasma peak concentration was 40% lower, AUC was decreased by 25% and time to peak plasma concentration was prolonged by 35 minutes. The clinical relevance of this decrease is unknown.
Distribution
Plasma protein binding is negligible. Metformin partitions into erythrocytes. The mean volume of distribution (Vd) ranged between 63 - 276 litres.
Biotransformation
Metformin is excreted unchanged in the urine. No metabolites have been identified in humans
Elimination
Metformin is eliminated by renal excretion. Renal clearance of Metformin is > 400 ml/min, indicating that Metformin is eliminated by glomerular filtration and tubular secretion. Following an oral dose, the apparent terminal elimination half-life is approximately 6.5 h. When renal function is impaired, renal clearance is decreased in proportion to that of creatinine and thus the elimination half-life is prolonged, leading to increased levels of Metformin in plasma.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Animal studies of up to 13-week duration have been conducted with the combined substances in Vil GM. No new toxicities associated with the combination were identified. The following data are findings from studies per formed with Vildagliptin or Metformin individually.
Vildagliptin
Intra-cardiac impulse conduction delays were observed in dogs with a no-effect dose of 15 mg/kg (7-fold human exposure based on Cmax). Accumulation of foamy alveolar macrophages in the lung was observed in rats and mice. The no-effect dose in rats was 25 mg/kg (5-fold human exposure based on AUC) and in mice 750 mg/kg (142-fold human exposure). Gastrointestinal symptoms, particularly soft faeces, mucoid faeces, diarrhoea and, at higher doses, faecal blood were observed in dogs. A no-effect level was not established. Vildagliptin was not mutagenic in conventional in vitro and in vivo tests for genotoxicity. A fertility and early embryonic development study in rats revealed no evidence of impaired fertility, reproductive performance or early embryonic development due to Vildagliptin. Embryo-foetal toxicity was evaluated in rats and rabbits. An increased incidence of wavy ribs was observed in rats in association with reduced maternal body weight parameters, with a no-effect dose of 75 mg/kg (10-fold human exposure). In rabbits, decreased foetal weight and skeletal variations indicative of developmental delays were noted only in the presence of severe maternal toxicity, with a no-effect dose of 50 mg/kg (9-fold human exposure). A pre- and postnatal development study was performed in rats. Findings were only observed in association with maternal toxicity at ≥ 150 mg/kg and included a transient decrease in body weight and reduced motor activity in the F1 generation. A two-year carcinogenicity study was conducted in rats at oral doses up to 900 mg/kg (approximately 200 times human exposure at the maximum recommended dose). No increases in tumour incidence attributable to Vildagliptin were observed. Another two-year carcinogenicity study was conducted in mice at oral doses up to 1,000 mg/kg. An increased incidence of mammary adenocarcinomas and haemangiosarcomas was observed with a no-effect dose of 500 mg/kg (59-fold human exposure) and 100 mg/kg (16-fold human exposure), respectively. The increased incidence of these tumours in mice is considered not to represent a significant risk to humans based on the lack of genotoxicity of Vildagliptin and its principal metabolite, the occurrence of tumours only in one species and the high systemic exposure ratios at which tumours were observed. In a 13-week toxicology study in cynomolgus monkeys, skin lesions have been recorded at doses ≥ 5 mg/kg/day. These were consistently located on the extremities (hands, feet, ears and tail). At 5 mg/kg/day (approximately equivalent to human AUC exposure at the 100 mg dose), only blisters were observed. They were reversible despite continued treatment and were not associated with histopathological abnormalities. Flaking skin, peeling skin, scabs and tail sores with correlating histopathological changes were noted at doses ≥ 20 mg/kg/day (approximately 3 times human AUC exposure at the 100 mg dose). Necrotic lesions of the tail were observed at ≥ 80 mg/kg/day. Skin lesions were not reversible in the monkeys treated at 160 mg/kg/day during a 4-week recovery period.
Metformin
Non-clinical data on Metformin reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential and toxicity to reproduction
7.0 Description
Vil GM tablet combines two antihyperglycaemic agents with complimentary mechanisms of action to improve glycaemic control in patients with type 2 diabetes : Vildagliptin, a member of the islet enhancer class, and Metformin Hydrochloride, a member of the biguanide class.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
Vil GM 500 : Alu-Alu blister strip of 10 tablets.
Vil GM 1000 : Alu-Alu blister strip of 10 tablets.
8.4 Storage and handing instructions
Store protected from moisture at a temperature not exceeding 30°C.
Keep out of reach of children.
9.0 Patient counselling information
Do not take Vil GM
- If you are allergic to Vildagliptin, Metformin or any of the other ingredients of this medicine. If you think you may be allergic to any of these, talk to your doctor before taking Vil GM.
- If you have uncontrolled diabetes, with, for example, severe hyperglycaemia (high blood glucose), nausea, vomiting, diarrhoea, rapid weight loss, lactic acidosis or ketoacidosis. Ketoacidosis is a condition in which substances called Ketone bodies accumulate in the blood and which can lead to diabetic pre-coma. Symptoms include stomach pain, fast and deep breathing, sleepiness or your breath developing an unusual fruity smell.
- If you have recently had a heart attack or if you have heart failure or serious problems with your blood circulation or difficulties in breathing which could be a sign of heart problems.
- If you have severely reduced kidney function.
- If you have a severe infection or are seriously dehydrated (have lost a lot of water from your body).
- If you are going to have a contrast x-ray (a specific type of x-ray involving an injectable dye).
- If you have liver problems.
- If you drink alcohol excessively (whether every day or only from time to time).
- If you are breast-feeding.
Warnings and precautions
Risk of lactic acidosis
Vil GM may cause a very rare, but very serious side effect called lactic acidosis, particularly if your kidneys are not working properly. The risk of developing lactic acidosis is also increased with uncontrolled diabetes, serious infections, prolonged fasting or alcohol intake, dehydration, liver problems and any medical conditions in which a part of the body has a reduced supply of oxygen (such as acute severe heart disease). If any of the above apply to you, talk to your doctor for further instructions.
Stop taking Vil GM for a short time
If you have a condition that may be associated with dehydration (significant loss of body fluids) such as severe vomiting, diarrhoea, fever, exposure to heat or if you drink less fluid than normal. Talk to your doctor for further instructions. Stop taking Vil GM and contact a doctor or the nearest hospital immediately if you experience some of the symptoms of lactic acidosis, as this condition may lead to coma. Symptoms of lactic acidosis include :
- Vomiting
- Stomach ache (abdominal pain)
- Muscle cramps
- A general feeling of not being well with severe tiredness
- Difficulty in breathing
- Reduced body temperature and heartbeat
Lactic acidosis is a medical emergency and must be treated in a hospital. Vil GM is not a substitute for insulin. Therefore, you should not receive Vil GM for the treatment of type 1 diabetes. Talk to your doctor, pharmacist or nurse before taking Vil GM if you have or have had a disease of the pancreas. Talk to your doctor, pharmacist or nurse before taking Vil GM if you are taking an anti-diabetic medicine known as a sulphonylurea. Your doctor may want to reduce your dose of the sulphonylurea when you take it together with Vil GM in order to avoid low blood glucose (hypoglycaemia). If you have previously taken vildagliptin but had to stop taking it because of liver disease, you should not take this medicine.
Diabetic skin lesions are a common complication of diabetes. You are advised to follow the recommendations for skin and foot care that you are given by your doctor or nurse. You are also advised to pay particular attention to new onset of blisters or ulcers while taking Vil GM. Should these occur, you should promptly consult your doctor. If you need to have major surgery you must stop taking Vil GM during and for some time after the procedure. Your doctor will decide when you must stop and when to restart your treatment with Vil GM. A test to determine your liver function will be performed before the start of Vil GM treatment, at three-month intervals for the first year and periodically thereafter. This is so that signs of increased liver enzymes can be detected as early as possible
During treatment with Vil GM, your doctor will check your kidney function at least once a year or more frequently if you are elderly and/or have worsening renal function. Your doctor will test your blood and urine for sugar regularly.
Children and adolescents
The use of Vil GM in children and adolescents up to 18 years of age is not recommended.
Other medicines and Vil GM
If you need to have an injection of a contrast medium that contains iodine into your bloodstream, for example in the context of an X-ray or scan, you must stop taking Vil GM before or at the time of the injection. Your doctor will decide when you must stop and when to restart your treatment with Vil GM. Tell your doctor if you are taking, have recently taken or might take any other medicines. You may need more frequent blood glucose and kidney function tests, or your doctor may need to adjust the dosage of Vil GM. It is especially important to mention the following :
- Glucocorticoids generally used to treat inflammation.
- Beta-2 agonists generally used to treat respiratory disorders.
- Other medicines used to treat diabetes.
- Medicines which increase urine production (diuretics).
- Medicines used to treat pain and inflammation (NSAID and COX-2-inhibitors, such as Ibuprofen and Celecoxib).
- Certain medicines for the treatment of high blood pressure (ACE inhibitors and angiotensin II receptor antagonists).
- Certain medicines affecting the thyroid.
- Certain medicines affecting the nervous system.
- Certain medicines used to treat angina (e.g. Ranolazine).
- Certain medicines used to treat HIV infection (e.g. Dolutegravir).
- Certain medicines used to treat a specific type of thyroid cancer (medullary thyroid cancer) (e.g. Vandetanib).
- Certain medicines used to treat heartburn and peptic ulcers (e.g Cimetidine).
Vil GM with alcohol
Avoid excessive alcohol intake while taking Vil GM since this may increase the risk of lactic acidosis.
Pregnancy and breast-feeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Your doctor will discuss with you the potential risk of taking Vil GM during pregnancy. Do not use Vil GM if you are pregnant or breast-feeding. Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
If you feel dizzy while taking Vil GM, do not drive or use any tools or machines.
When and how to take Vil GM
Swallow the tablets whole with a glass of water. Take one tablet in the morning and the other in the evening with or just after food. Taking the tablet just after food will lower the risk of an upset stomach. Continue to follow any advice about diet that your doctor has given you. In particular, if you are following a diabetic weight control diet, continue with this while you are taking Vil GM.
If you take more Vil GM than you should
If you take too many Vil GM tablets, or if someone else takes your tablets, talk to a doctor or pharmacist immediately. Medical attention may be necessary. If you have to go to a doctor or hospital, take the pack and this leaflet with you.
If you forget to take Vil GM
If you forget to take a tablet, take it with your next meal unless you are due to take one then anyway. Do not take a double dose (two tablets at once) to make up for a forgotten tablet.
If you stop taking Vil GM
Continue to take this medicine as long as your doctor prescribes it so that it can continue to control your blood sugar. Do not stop taking Vil GM unless your doctor tells you to. If you have any questions about how long to take this medicine, talk to your doctor.
12.0 Date of revision
10 November 2022
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What VIL GM is and what it is used for
- What you need to know before you take VIL GM
- How to take VIL GM
- Possible side effects
- How to store VIL GM
- Contents of the pack and other information
1. What Vil Gm is and What It is Used for
The active substances of VIL GM, vildagliptin and metformin, belong to a group of medicines called “oral antidiabetics”. VIL GM is used to treat adult patients with type 2 diabetes. This type of diabetes is also known as noninsulin-dependent diabetes mellitus. Type 2 diabetes develops if the body does not make enough insulin or if the insulin that the body makes does not work as well as it should. It can also develop if the body produces too much glucagon. Both insulin and glucagon are made in the pancreas. Insulin helps to lower the level of sugar in the blood, especially after meals. Glucagon triggers the liver to make sugar, causing the blood sugar level to rise.
How VIL GM works
Both active substances, vildagliptin and metformin, help to control the level of sugar in the blood. The substance vildagliptin works by making the pancreas produce more insulin and less glucagon. The substance metformin works by helping the body to make better use of insulin. This medicine has been shown to reduce blood sugar, which may help to prevent complications from your diabetes.
Even though you are now starting a medicine for your diabetes, it is important that you continue to follow the diet and/or exercise which has been recommended for you.
2. What You Need to Know Before You Take Vil Gm
Do not use VIL GM
if you are allergic to vildagliptin, metformin or any of the other ingredients of this medicine.
If you think you may be allergic to any of these, talk to your doctor before taking VIL GM.
if you have uncontrolled diabetes, with, for example, severe hyperglycaemia (high blood glucose), nausea, vomiting, diarrhoea, rapid weight loss, lactic acidosis or ketoacidosis. Ketoacidosis is a condition in which substances called ketone bodies accumulate in the blood and which can lead to diabetic pre-coma. Symptoms include stomach pain, fast and deep breathing, sleepiness or your breath developing an unusual fruity smell.
if you have recently had a heart attack or if you have heart failure or serious problems with your blood circulation or difficulties in breathing which could be a sign of heart problems.
if you have severely reduced kidney function. if you have a severe infection or are seriously dehydrated (have lost a lot of water from your body).
if you are going to have a contrast x-ray (a specific type of x-ray involving an injectable dye). Please also see information about this in section “Warnings and precautions”.
if you have liver problems. if you drink alcohol excessively (whether every day or only from time to time).
if you are breast-feeding (see also “Pregnancy and breast-feeding”).
Warnings and precautions
Risk of lactic acidosis
VIL GM may cause a very rare, but very serious side effect called lactic acidosis, particularly if your kidneys are not working properly. The risk of developing lactic acidosis is also increased with uncontrolled diabetes, serious infections, prolonged fasting or alcohol intake, dehydration (see further information below), liver problems and any medical conditions in which a part of the body has a reduced supply of oxygen (such as acute severe heart disease). If any of the above apply to you, talk to your doctor for further instructions.
Stop taking VIL GM for a short time if you have a condition that may be associated with dehydration (significant loss of body fluids) such as severe vomiting, diarrhoea, fever, exposure to heat or if you drink less fluid than normal. Talk to your doctor for further instructions.
Stop taking VIL GM and contact a doctor or the nearest hospital immediately if you experience some of the symptoms of lactic acidosis, as this condition may lead to coma. Symptoms of lactic acidosis include:
- vomiting
- stomach ache (abdominal pain)
- muscle cramps
- a general feeling of not being well with severe tiredness
- difficulty in breathing
- reduced body temperature and heartbeat
Lactic acidosis is a medical emergency and must be treated in a hospital.
VIL GM is not a substitute for insulin. Therefore, you should not receive VIL GM for the treatment of type 1 diabetes. Talk to your doctor, pharmacist or nurse before taking VIL GM if you have or have had a disease of the pancreas. Talk to your doctor, pharmacist or nurse before taking VIL GM if you are taking an anti-diabetic medicine known as a sulphonylurea. Your doctor may want to reduce your dose of the sulphonylurea when you take it together with VIL GM in order to avoid low blood glucose (hypoglycaemia). If you have previously taken vildagliptin but had to stop taking it because of liver disease, you should not take this medicine. Diabetic skin lesions are a common complication of diabetes. You are advised to follow the recommendations for skin and foot care that you are given by your doctor or nurse. You are also advised to pay particular attention to new onset of blisters or ulcers while taking VIL GM. Should these occur, you should promptly consult your doctor. If you need to have major surgery you must stop taking VIL GM during and for some time after the procedure. Your doctor will decide when you must stop and when to restart your treatment with VIL GM. A test to determine your liver function will be performed before the start of VIL GM treatment, at three-month intervals for the first year and periodically thereafter. This is so that signs of increased liver enzymes can be detected as early as possible. During treatment with VIL GM, your doctor will check your kidney function at least once a year or more frequently if you are elderly and/or have worsening renal function. Your doctor will test your blood and urine for sugar regularly
Children and adolescents
The use of VIL GM in children and adolescents up to 18 years of age is not recommended.
Other medicines and VIL GM
If you need to have an injection of a contrast medium that contains iodine into your bloodstream, for example in the context of an X-ray or scan, you must stop taking VIL GM before or at the time of the injection. Your doctor will decide when you must stop and when to restart your treatment with VIL GM.
Tell your doctor if you are taking, have recently taken or might take any other medicines. You may need more frequent blood glucose and kidney function tests, or your doctor may need to adjust the dosage of VIL GM. It is especially important to mention the following:
- glucocorticoids generally used to treat inflammation
- beta-2 agonists generally used to treat respiratory disorders
- other medicines used to treat diabetes
- medicines which increase urine production (diuretics)
- medicines used to treat pain and inflammation (NSAID and COX-2-inhibitors, such as ibuprofen and celecoxib)
- certain medicines for the treatment of high blood pressure (ACE inhibitors and angiotensin II receptor antagonists)
- certain medicines affecting the thyroid, or
- certain medicines affecting the nervous system.
VIL GM with alcohol
Avoid excessive alcohol intake while taking VIL GM since this may increase the risk of lactic acidosis (please see section “Warnings and precautions”).
Pregnancy and breast-feeding
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Your doctor will discuss with you the potential risk of taking VIL GM during pregnancy. Do not use VIL GM if you are pregnant or breast-feeding (see also “Do not take VIL GM”). Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
If you feel dizzy while taking VIL GM, do not drive or use any tools or machines.
3. How to Use Vil Gm
The amount of VIL GM that people have to take varies depending on their condition. Your doctor will tell you exactly the dose of VIL GM to take.
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The recommended dose is one film-coated tablet of either 50 mg/5000 mg or 50 mg/1000 mg taken twice a day If you have reduced kidney function, your doctor may prescribe a lower dose. Also if you are taking an anti-diabetic medicine known as a sulphonylurea your doctor may prescribe a lower dose. Your doctor may prescribe this medicine alone or with certain other medicines that lower the level of sugar in your blood. When and how to take VIL GM
- Swallow the tablets whole with a glass of water,
- Take one tablet in the morning and the other in the evening with or just after food. Taking the tablet just after food will lower the risk of an upset stomach.
Continue to follow any advice about diet that your doctor has given you. In particular, if you are following a diabetic weight control diet, continue with this while you are taking VIL GM.
If you take more VIL GM than you should
If you take too many VIL GM tablets, or if someone else takes your tablets, talk to a doctor or pharmacist immediately. Medical attention may be necessary. If you have to go to a doctor or hospital, take the pack and this leaflet with you.
If you forget to take VIL GM
If you forget to take a tablet, take it with your next meal unless you are due to take one then anyway.
Do not take a double dose (two tablets at once) to make up for a forgotten tablet.
If you stop taking VIL GM
Continue to take this medicine as long as your doctor prescribes it so that it can continue to control your blood sugar. Do not stop taking VIL GM unless your doctor tells you to. If you have any questions about how long to take this medicine, talk to your doctor. If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You should stop taking VIL GM and see your doctor immediately if you experience the following side effects:
- Lactic acidosis (very rare: may affect up to 1 user in 10,000): VIL GM may cause a very rare, but very serious side effect called lactic acidosis (see section “Warnings and precautions”). If this happens you must stop taking VIL GM and contact a doctor or the nearest hospital immediately, as lactic acidosis may lead to coma.
- Angioedema (rare: may affect up to 1 in 1,000 people): Symptoms include swollen face, tongue or throat, difficulty swallowing, difficulty breathing, sudden onset of rash or hives, which may indicate a reaction called “angioedema”.
- Liver disease (hepatitis) (rare): Symptoms include yellow skin and eyes, nausea, loss of appetite or dark-coloured urine, which may indicate liver disease (hepatitis).
- Inflammation of the pancreas (pancreatitis) (frequency not known): Symptoms include severe and persistent pain in the abdomen (stomach area), which might reach through to your back, as well as nausea and vomiting.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to Store Vil Gm
Store protect from moisture, at a temperature not exceeding 30°C.
Keep out of reach of children.
Do not use this medicine after the expiry date which is stated on the blister and the carton after “EXP”. The expiry date refers to the last day of that month.
6. Contents of the Pack and Other Information
What VIL GM contains
Vil GM 500
Each film coated tablet contains
Vildagliptin 50 mg
Metformin hydrochloride 500 mg
Excipients q.s.
Colours : Yellow Oxide of Iron and Titanium Dioxide IP
Vil GM 1000
Each film coated tablet contains Vildagliptin 50 mg
Metformin hydrochloride 1000 mg
Excipients q.s.
Colours : Yellow Oxide of Iron and Titanium Dioxide IP
© Zuventus Healthcare Ltd., 2020. All rights reserved.