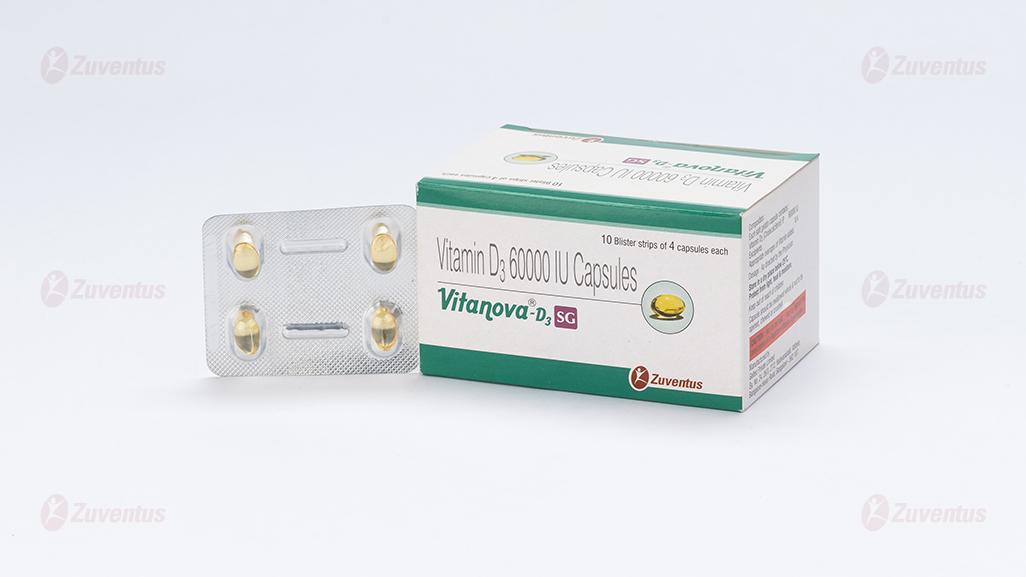
Vitanova-D3 SG Capsule
Therapy Area
Vitamins/Minerals Supplements
1. Generic Name
Cholecalciferol (Vitamin-D3) Capsules
2. Qualitative and quantitative composition
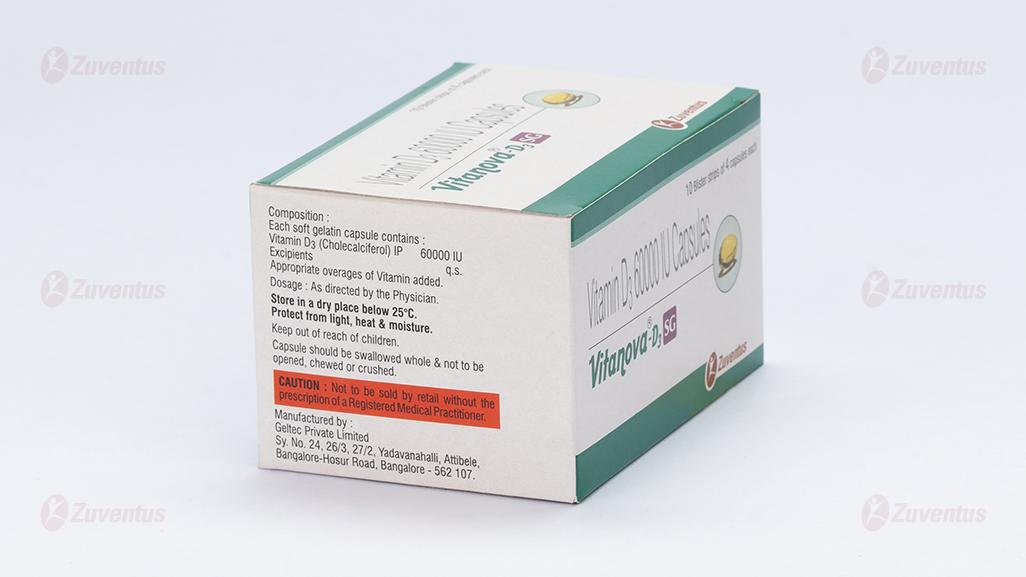
Each soft gelatin capsules contains:
Cholecalciferol (Vitamin D3) IP 1500 mcg
equivalent to 60,000 IU
Excipients q.s.
Appropriate overages of vitamin added to compensate the loss on storage.
3. Dosage form and strength
Soft Gelatin Capsules
60,000 IU
4. Clinical particulars
4.1 Therapeutic indication
For the treatment of vitamin D3 deficiency
4.2 Posology and method of administration
Adults and adolescent: One Vitanova-D3 SG Capsules (60,000 IU) to be given once a week for a period of 8 weeks, followed by one Vitanova-D3 SG Capsules (60,000 IU) once a month or daily maintenance dose as directed by the physician.
Certain populations are at high risk of vitamin D deficiency, and may require higher doses and monitoring of serum 25(OH)D:
- Institutionalised or hospitalised individuals
- Dark skinned individuals
- Individuals with limited effective sun exposure due to protective clothing or consistent use of sun screens
- Obese individuals
- Patients being evaluated for osteoporosis
- Use of certain concomitant medications (e.g., anticonvulsant medications, glucocorticoids)
- Patients with malabsorption, including inflammatory bowel disease and coeliac disease
- Those recently treated for vitamin D deficiency, and requiring maintenance therapy.
Infants and young children (0-12 years)
Vitanova-D3 SG Capsules should not be given to children under 12 years due to the risk of choking.
Method of administration
This medicine is taken orally.
The Vitanova-D3 SG capsule should be swallowed whole with water, preferably with the main meal of the day.
4.3 Contraindications
- Hypersensitivity to vitamin D or any of the excipients in the product
- Hypervitaminosis D
- Nephrolithiasis
- Diseases or conditions resulting in hypercalcaemia and/or hypercalciuria
- Severe renal impairment
4.4 Special warnings and precautions for use
- Vitamin D should be used with caution in patients with impairment of renal function and the effect on calcium and phosphate levels should be monitored. The risk of soft tissue calcification should be taken into account. In patients with severe renal insufficiency, vitamin D in the form of Cholecalciferol is not metabolised normally and other forms of vitamin D should be used.
- Caution is required in patients receiving treatment for cardiovascular disease.
- Vitanova-D3 should be prescribed with caution to patients suffering from sarcoidosis because of the risk of increased metabolism of vitamin D to its active form. These patients should be monitored with regard to the calcium content in serum and urine.
- Allowances should be made for vitamin D supplements from other sources.
- The need for additional calcium supplementation should be considered for individual patients. Calcium supplements should be given under close medical supervision.
- Medical supervision is required whilst on treatment to prevent hypercalcaemia.
4.5 Drugs interactions
- Concomitant treatment with phenytoin or barbiturates can decrease the effect of vitamin D because of metabolic activation. Concomitant use of glucocorticoids can decrease the effect of vitamin D.
- The effects of digitalis and other cardiac glycosides may be accentuated with the oral administration of calcium combined with Vitamin D. Strict medical supervision is needed and, if necessary monitoring of ECG and calcium.
- Simultaneous treatment with ion exchange resins such as cholestyramine or laxatives such as paraffin oil may reduce the gastrointestinal absorption of vitamin D.
- The cytotoxic agent actinomycin and imidazole antifungal agents interfere with vitamin D activity by inhibiting the conversion of 25-hydroxyvitamin D to 1,25- dihydroxyvitamin D by the kidney enzyme, 25-hydroxyvitamin D-1-hydroxylase.
4.6 Use in special populations
Pregnancy
No data are available for cholecalciferol (vitamin D3). There are no studies in pregnant women. It should be used during pregnancy only if the potential benefit justifies the potential risk to the mother and fetus.
Nursing Mothers
Cholecalciferol and some of its active metabolites pass into breast milk. Infants should be closely monitored for hypercalcemia or clinical manifestations of vitamin D toxicity if the mother is taking pharmacological doses of vitamin D3.
Infants
Vitamin D3 should be used with caution in infants, who may have increased sensitivity to its effects.
4.7 Effects on ability to drive and use machines
Vitanova D3 has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Adverse reactions are listed below, by system organ class and frequency. Frequencies are defined as: uncommon (>1/1,000, <1/100) or rare (>1/10,000, <1/1,000).
Metabolism and nutrition disorders
Uncommon: Hypercalcaemia and hypercalciuria. Skin and subcutaneous disorders
Rare: Pruritus, rash and urticaria.
Reporting of suspected adverse reactions
- Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
- By reporting side effects, you can help provide more information on the safety of this medicine.
4.8 Overdose
The most serious consequence of acute or chronic overdose is hypercalcaemia due to vitamin D toxicity. Symptoms may include nausea, vomiting, polyuria, anorexia, weakness, apathy, thirst and constipation. Chronic overdoses can lead to vascular and organ calcification as a result of hypercalcaemia.
Treatment should consist of stopping all intake of vitamin D and rehydration. Rehydration, and, according to severity, isolated or combined treatment with loop diuretics, bisphosphonates, calcitonin and corticosteroids should be considered. Serum electrolytes, renal function and diuresis must be monitored.
5. Pharmacological properties
5.1 Mechanism of Action
In its biologically active form vitamin D3 stimulates intestinal calcium absorption, incorporation of calcium into the osteoid, and release of calcium from bone tissue. In the small intestine it promotes rapid and delayed calcium uptake. The passive and active transport of phosphate is also stimulated. In the kidney, it inhibits the excretion of calcium and phosphate by promoting tubular resorption. The production of parathyroid hormone (PTH) in the parathyroids is inhibited directly by the biologically active form of vitamin D3. PTH secretion is inhibited additionally by the increased calcium uptake in the small intestine under the influence of biologically active vitamin D3.
5.2 Pharmacodynamic properties
Vitamin D3 is converted to 25-hydroxyvitamin D3 in the liver. Conversion to the active calcium-mobilizing hormone 1, 25-dihydroxyvitamin D3 (calcitriol) in the kidney is stimulated by both parathyroid hormone and hypophosphatemia. The principal action of 1,25-dihydroxyvitamin D3 is to increase intestinal absorption of both calcium and phosphate as well as regulate serum calcium, renal calcium and phosphate excretion, bone formation and bone resorption.
Vitamin D is required for normal bone formation. Vitamin D insufficiency develops when both sunlight exposure and dietary intake are inadequate. Insufficiency is associated with negative calcium balance, increased parathyroid hormone levels, bone loss, and increased risk of skeletal fracture. In severe cases, deficiency results in more severe hyperparathyroidism, hypophosphatemia, proximal muscle weakness, bone pain and osteomalacia.
5.3 Pharmacokinetic properties
The pharmacokinetics of vitamin D is well known. Vitamin D is well absorbed from the gastro-intestinal tract in the presence of bile. It is hydroxylated in the liver to form 25-hydroxyCholecalciferol and then undergoes further hydroxylation in the kidney to form the active metabolite 1, 25 dihydroxy Cholecalciferol (calcitriol). The metabolites circulate in the blood bound to a specific α - globin, Vitamin D and its metabolites are excreted mainly in the bile and faeces.
6. Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Vitamin D is well known and is a widely used material and has been used in clinical practice for many years. As such toxicity is only likely to occur in chronic overdosage where hypercalcaemia could result.
Cholecalciferol has been shown to be teratogenic in high doses in animals (4-15 times the human dose). Offspring from pregnant rabbits treated with high doses of vitamin D had lesions anatomically similar to those of supravalvular aortic stenosis and offspring not showing such changes show vasculotoxicity similar to that of adults following acute vitamin D toxicity.
7. Description
Vitanova D3 contains cholecalciferol (Vitamin D3). Vitamin D3 is essential for the proper growth and development of the body. It is synthesized within the body after exposure to sunlight and is essential for many important functions of the human body. Vitamin D3 in Vitanova D3 also increases the Calcium absorption from the intestines.
8. Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on the pack
8.3 Packaging information
10 Blister strips of 4 capsules each
8.4 Storage and handing instructions
Store at a temperature not exceeding 30°C. Protect from direct sunlight.
Keep out of reach of children
Capsule should be swallowed whole & not to be opened, chewed or crushed
9. Patient Counselling Information
- Take exactly as directed by your doctor or on the label.
- Do not increase the dosage or take for longer than is recommended.
- Vitanova-D3 Capsules may interfere with cholesterol tests, hence please inform your physician and laboratory staff that you are taking Vitanova-D3 Capsules before undergoing blood tests.
- Clinical monitoring of serum electrolyte concentrations and cardiac function is recommended.
About leaflet
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What VITANOVA- D3 SG is and what it is used for
- What you need to know before you use VITANOVA- D3 SG
- How to use VITANOVA- D3 SG
- Possible side effects
- How to store VITANOVA- D3 SG
- Contents of the pack and other information
1. What VITANOVA- D3 SG is and what it is used for
VITANOVA- D3 SG capsules contains the active ingredient cholecalciferol (vitamin D3). Vitamin D3, can be found in some foods and is also produced by the body when skin is exposed to sunlight. Vitamin D3 helps the kidneys and intestine absorb calcium and it helps build bones. Vitamin D3 deficiency is the predominant cause of rickets (defective mineralization of bones in children) and osteomalacia (inadequate mineralization of bones in adults).
Certain populations are at high risk of vitamin D deficiency, and may require higher doses and monitoring of serum 25(OH)D:
- Institutionalised, foster homes or hospitalised individuals
- Dark skinned individuals
- Individuals with limited effective sun exposure due to protective clothing or consistent use of sun screens
- Obese individuals
- Patients being evaluated for osteoporosis
- Use of certain concomitant medications (e.g., anticonvulsant medications, glucocorticoids)
- Patients with malabsorption, including inflammatory bowel disease and coeliac disease
- Those recently treated for vitamin D deficiency, and requiring maintenance therapy.
VITANOVA- D3 soft capsules is used for initial treatment of clinically relevant vitamin D deficiency in adults.
2. What you need to know before you use VITANOVA- D3 SG
Do not use VITANOVA- D3:
- if you are allergic to vitamin D3 or any of the other ingredients of this medicine;
- if you have high levels of calcium in your blood (hypercalcaemia) or urine (hypercalciuria);
- if you have kidney stones (renal calculi) or severe renal impairment;
- if you have high levels of vitamin D3 in your blood (hypervitaminosis D)
Warnings and precautions
- Talk to your doctor, pharmacist or nurse before using VITANOVA- D3 if you:
- are undergoing treatment with certain medicines used to treat heart disorders (e.g., cardiac glycosides, such as digoxin);
- have sarcoidosis (an immune system disorder which may cause increased levels of vitamin D3 in the body);
- are treated with diuretics (e.g. benzothiadiazine)
- are immobilized
- suffer from pseudohypoparathyroidism
- are taking medicines containing vitamin D3, or eating foods or milk enriched with vitamin D3;
- are likely to be exposed to a lot of sunshine whilst using VITANOVA- D3;
- take additional supplements containing calcium. Your doctor will monitor your blood levels of calcium to make sure they are not too high whilst you are using VITANOVA- D3;
- have kidney damage or disease and if you have a tendency for the formation of renal stones. Your doctor may want to measure the levels of calcium in your blood or urine. take a daily dose of vitamin D3 exceeding 1,000 I.U. over a long period of time, your doctor should monitor the level of calcium in your blood by lab test.
Children and Adolescents
The use is not recommended in children under 12 years of age.
Other medicines and VITANOVA- D3
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines. This is especially important if you are taking:
- medicines that act on the heart or kidneys, such as cardiac glycosides (eg, digoxin) or diuretics (e.g., bendroflumethiazide). When used at the same time as vitamin D3 these medicines may cause a large increase in the level of calcium in the blood and urine;
- medicines containing vitamin D3 or eating food rich in vitamin D3, such as, some types of vitamin D3-enriched milk;
- actinomycin (a medicine used to treat some forms of cancer) and imidazole antifungals (eg, clotrimazole and ketoconazole, medicines used to treat fungal disease). These medicines may interfere with the way your body process vitamin D3;
- medicines to treat tuberculosis e.g rifampicin, isoniazid;
- the following medicines because they can interfere with the effect or the absorption of vitamin D3:
- antiepileptic medicines (anticonvulsants), barbiturates;
- glucocorticoids (steroid hormones such as hydrocortisone or prednisolone). These can decrease the effect of vitamin D3;
- medicines that lower the level of cholesterol in the blood (such as cholestyramine, or colestipol);
- certain medicines for weight loss that reduce the amount of fat your body absorbs (eg, orlistat); certain laxatives (such as liquid paraffin).
VITANOVA- D3 SG with food, drink and alcohol
You should take this medicine preferably together with a meal to help your body absorb the vitamin D3.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. This high strength formulation is not recommended for use in pregnant and breastfeeding women.
Driving and using machines
VITANOVA- D3 SG should not affect your ability to drive or operate machinery.
3. How to use VITANOVA- D3 SG
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure. This medicine is taken orally.
The capsule should be swallowed whole (e.g. with water), not to be opened, chewed or crushed.
You should take Vitanova-D3 SG preferably together with a meal.
Adults and adolescent: One Vitanova-D3 SG Capsules (60,000 IU) to be given once a week for a period of 8 weeks, followed by one Vitanova-D3 SG Capsules (60,000 IU) once a month or daily maintenance dose as directed by the physician.
Your doctor will adjust the dose for you.
Pediatric population
Vitanova-D3 SG 60, 000 I.U. is not recommended in children under 12 years of age.
Pregnancy and breastfeeding
Vitanova-D3 SG should be used during pregnancy and lactation only if the potential benefit justifies the potential risk to the mother and fetus.
If you are pregnant or think you may be pregnant or you are breast-feeding, you should talk to your doctor or pharmacist before you take Vitanova-D3 SG.
If you take more VITANOVA- D3 SG than you should
If you take more medicine than prescribed, stop using this medicine and contact your doctor. If it is not possible to talk to a doctor go to the nearest hospital emergency department and take the medicine package with you. The most common symptoms of overdose are: nausea, vomiting, excessive thirst, the production of large amounts of urine over 24 hours, constipation and dehydration, high levels of calcium in the blood and in urine (hypercalcaemia and hypercalciuria) shown by lab test.
If you forget to take Vitanova- D3 SG
If you forget to take a dose of Vitanova- D3, take the forgotten dose as soon as possible. Then take the next dose at the correct time. However, if it is almost time to take the next dose, do not take the dose you have missed; just take the next dose as normal. Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Possible side effects may include:
Uncommon (affects less than 1 in 100 people)
- Too much calcium in your blood (hypercalcaemia)
- Too much calcium in your urine (hypercalciuria)
Rare (affects less than 1 in 1000 people)
- Skin rash
- Itching
- Hives
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store VITANOVA- D3 SG
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton and blister pack after "Exp". The expiry date refers to the last day of that month. Do not store above 30° C.
Store in the original package in order to protect from light. Do not freeze. Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What VITANOVA- D3 contains
The active substance is cholecalciferol (vitamin D3). One capsule contains: 1500 mcg cholecalciferol (vitamin D3) equivalent to 60, 000 IU.
Packaging
10 Blister strips of 4 capsules each