Vitanova®-D3 6L Injection
1.0 Generic Name
Cholecalciferol Injection IP
2.0 Qualitative and quantitative composition
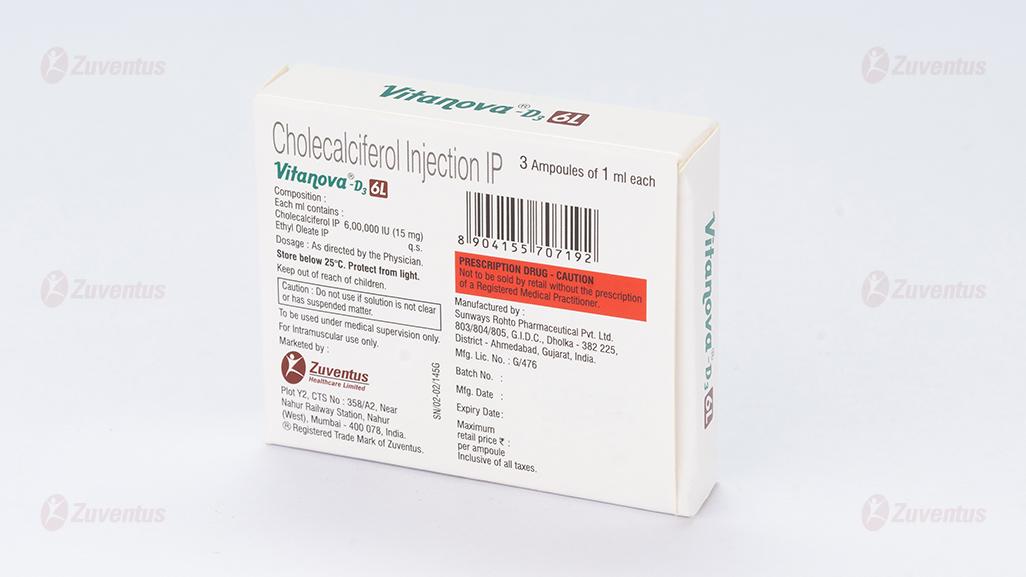
Each ml contains:
Cholecalciferol IP 6,00,000 IU (15mg)
Ethyl Oleate IP q.s.
3.0 Dosage form and strength
Ampoule, 6,00,000 IU
For intramuscular use only.
4.0 Clinical particulars
4.1 Therapeutic Indication
Indicated for the treatment of Vitamin D3 deficiency
4.2 Posology and method of administration
Vitamin D3 injection is administered intramuscularly as a single dose of 6,00,000 IU once only or repeated after 6 months to 1 year, depending upon clinical response and requirements. The serum calcium levels should be checked every 3 - 6 months and the dose adapted according to the values.
4.3 Contraindications
- Hypersensitivity to the active substance(s) or to any of the excipients.
- Hypercalcaemia, evidence of vitamin D toxicity, hypervitaminosis D, decreased renal function, metastatic calcification.
4.4 Special warnings and precautions for use
- Adequate dietary calcium is necessary for clinical response to Cholecalciferol therapy.
- Caution should be used when the injectable forms are used in patients with vitamin D resistant rickets as the range between the toxic and therapeutic dosage is narrow.
- Vitamin D should be administered with caution to infants and patients who may have an increased sensitivity to its effects. Use with care in patients with renal impairment, renal calculi or heart disease or arteriosclerosis who might be at increased risk of organ damage if hypercalcaemia were to occur.
- Cholecalciferol is not recommended for use in hypoparathyroidism. In the event of hypoparathyroidism when Cholecalciferol is used, calcium, parathyroid hormone or dihydrotachysterol may be required.
- Dosage should be individualised. Frequent serum and urinary calcium, phosphate and urea nitrogen determinations should be carried out. Adequate fluid intake should be maintained.
- Should hyperglycaemia develop, Cholecalciferol should be discontinued immediately.
- Because of the effect on serum calcium, Cholecalciferol should only be administered to patients with renal stones when potential benefits outweigh possible hazards.
- Vitamin D3 supplementation may worsen hypercalcemia and/or hypercalciuria when administered to patients with diseases associated with unregulated overproduction of calcitriol (e.g., leukemia, lymphoma, sarcoidosis). Urine and serum calcium should be monitored in these patients.
- It should be used with caution in patients with renal impairment or calculi, heart disease, sarcoidosis, pseudo hypoparathyroidism and who might be at increased risk of organ damage if hypercalcemia occurred.
- Plasma calcium and phosphate concentrations should be controlled during vitamin D3 therapy to reduce the risk of ectopic calcification.
4.5 Drugs interactions
- Rifampicin and isoniazid may reduce the effectiveness of vitamin D3.
- Corticosteroids may counteract the effect of vitamin D3. Cholecalciferol and
- Magnesium-containing antacids: hypermagnesaemia may develop in patients on chronic renal dialysis.
- Digitalis glycosides: hypercalcaemia in patients on digitalis may precipitate cardiac arrhythmias.
- Verapamil: atrial fibrillation has recurred when supplemental calcium and Cholecalciferol have induced hypercalcaemia.
- Anti-convulsants: Vitamin D requirements may be increased in patients taking anti-convulsants (e.g. carbamazepine, phenobarbital, phenytoin and primidone).
- Thiazide diuretics: hypoparathyroid patients on Cholecalciferol may develop hypercalcaemia.
4.6 Use in special populations
- Pregnancy :- There are no or limited amount of data from the use of ergocalciferol in pregnant women. Cholecalciferol Injection should not be used in pregnancy unless the potential benefit outweighs the potential hazards to the foetus.
- Nursing Mothers :- Vitamin D3 and some of its active metabolites pass into breast milk. Infants should be closely monitored for hypercalcemia or clinical manifestations of vitamin D toxicity if the mother is taking pharmacological doses of vitamin D3.
- Infants:- Vitamin D3 should be used with caution in infants.
- Elderly: Requirements of vitamin D3 are increased in the elderly patients.
- Renal Insufficiency:- Patients with renal insufficiency will have decreased ability to form calcitriol metabolite, the effect on the calcium and phosphate balance should be supervised.
4.7 Effects on ability to drive and use machines
Cholecalciferol injection have no influence on the ability to drive and use machines. If patient feels drowsiness, affected patients should not drive or operate machinery.
4.8 Undesirable effects
Adverse events are generally associated with excessive intake of Cholecalciferol leading to the development of hypercalcaemia.
Signs and symptoms of vitamin D intoxication associated with hypercalcemia include muscle weakness, apathy, headache, anorexia, nausea, vomiting, bone pain, ectopic calcification, proteinuria, hypertension and cardiac arrhythmias. Chronic hypercalcemia can lead to generalized vascular calcification, nephrocalcinosis, and rapid deterioration of renal function.
| System Organ Class | Adverse event | Frequency |
|---|---|---|
| Metabolism and nutrition disorders | Hypercalcaemia | Very common |
| Hypercholesterolaemia† | Not known | |
| Muscle weakness§ | Not known | |
| Muscle pain§ | Not known | |
| Mild acidosis† | Not known | |
| Polydipsia† | Not known | |
| Anorexia† | Not known | |
| Psychiatric disorders | Overt psychosis† | Rare |
| Somnolence§ | Not known | |
| Nervous system disorders | Headache§ | Not known |
| Endocrine disorders | Hypoparathyroidism* pseudohypopathyroidism* | Very common |
| Eye disorders | Conjunctivitis (calcific) | Not known |
| Photophobia | Not known | |
| Cardiac disorders | Cardiac arrhythmias | Not known |
| Rebal disorders | Elevated serum creatinine levels* | Very common |
| Vascular disorders | Generalised vascular calcification† | Not known |
| Hypertension† | Not known | |
| Respiratory, thoracic and mediastinal disorders | Rhinorrhoea† | Not known |
| Gastrointestinal disorders | Pancreatitis† | Not known |
| Nausea§ | Not known | |
| Vomiting§ | Not known | |
| Dry mouth§ | Not known | |
| Constipation§ | Not known | |
| Diarrhoea§ | Not known | |
| Abdominal pain§ | Not known | |
| Skin and subcutaneous tissue disorders | Pruritus† | Not known |
| Musculoskeletal and connective tissue disorders | Bone pain§ | Not known |
| Ectopic calcification† | Not known | |
| Renal and urinary disorders | Polyuria† | Not known |
| Nocturia† | Not known | |
| Nephrocalcinosis† | Not known | |
| Albuminuria† | Not known | |
| Reversible azotemia† | Not known | |
| Reproductive system and breast disorders | Decreased libido† | Not known |
| General disorders and administration site conditions | Hyperthermia† | Not known |
| Fatigue§ | Not known | |
| Irritability† | Not known | |
| Weakness§ | Not known | |
| Investigations | Elevated AST † | Not known |
| Elevated ALT† | Not known | |
| Elevated BUN† | Not known | |
| Weight loss† | Not known | |
| Surgical and medical procedures | Metallic taste§ | Not known |
*In clinical studies on hypoparathyroidism and pseudohypopathyroidism, hypercalcaemia was noted on at least one occasion in about 1 in 3 patients and hypercalciuria in about 1 in 7. Elevated serum creatinine levels were observed in about 1 in 6 patients (approximately one half of whom had normal levels at baseline).
§ Possible early symptoms of hypercalcaemia
†Possible late symptoms of hypercalcaemia
Reporting of suspected adverse reactions
- Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: www.medico@zuventus.com
- Website: http://www.zuventus.co.in/safety.aspx
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Symptoms
Administration to patients in excess of their daily requirement can cause hypercalcaemia, hypercalciuria and hyperphosphataemia. Concomitant high intake of calcium and phosphate may lead to similar abnormalities.
Management
Treatment of chronic overdose with resulting hypercalcaemia consists of immediate withdrawal of the vitamin, a low calcium diet and generous fluid intake. Severe cases may require hydration with intravenous saline together with symptomatic and supportive treatment as indicated by the patient's clinical condition. Plasma calcium should be monitored.
5.0 Pharmacological properties
5.1 Mechanism of Action
In its biologically active form vitamin D3 stimulates intestinal calcium absorption, incorporation of calcium into the osteoid, and release of calcium from bone tissue. In the small intestine it promotes rapid and delayed calcium uptake. The passive and active transport of phosphate is also stimulated. In the kidney, it inhibits the excretion of calcium and phosphate by promoting tubular resorption. The production of parathyroid hormone (PTH) in the parathyroids is inhibited directly by the biologically active form of vitamin D3. PTH secretion is inhibited additionally by the increased calcium uptake in the small intestine under the influence of biologically active vitamin D3.
The mechanism of action of 1,25(OH)2D (calcitriol) is mediated by the interaction of calcitriol with the vitamin D receptor (VDR). Calcitriol binds to cytosolic VDRs within target cells, and the receptor-hormone complex translocates to the nucleus and interacts with DNA to modify gene transcription. The VDR belongs to the steroid and thyroid hormone receptor supergene family. Calcitriol also exerts nongenomic effects that may require the presence of a functional VDR.
5.2 Pharmacodynamic properties
Vitamin D3 is converted to 25‑hydroxyvitamin D3 in the liver. Conversion to the active calcium-mobilizing hormone 1,25-dihydroxyvitamin D3 (calcitriol) in the kidney is stimulated by both parathyroid hormone (PTH) and hypophosphatemia. The known sites of action of calcitriol are intestine, bone, kidney and parathyroid gland. The principal action of calcitriol is to increase intestinal absorption of both calcium and phosphate as well as regulate serum calcium, renal calcium and phosphate excretion, bone formation and bone resorption.
Vitamin D is required for normal bone formation. Vitamin D insufficiency develops when both sunlight exposure and dietary intake are inadequate. Insufficiency is associated with negative calcium balance, increased PTH levels, bone loss, and increased risk of skeletal fracture. In severe cases, deficiency results in more severe hyperparathyroidism, hypophosphatemia, proximal muscle weakness, bone pain and osteomalacia.
5.3 Pharmacokinetic properties
After absorption, Vitamin D3 is rapidly distributed to the liver and lesser amount distributed to adipose tissue, and stored as vitamin D3 at these sites for later release into the circulation. Circulating vitamin D3 is bound to vitamin D-binding protein.
Vitamin D3 is rapidly metabolized by hydroxylation in the liver to 25-hydroxyvitamin D3 (calcidiol), and subsequently metabolized in the kidney to calcitriol, which represents the biologically active form. Further hydroxylation occurs prior to elimination. A small percentage of vitamin D3 undergoes glucuronidation prior to elimination.
The mean urinary excretion of Vitamin D3 after 48 hours is 2.4% of the administered dose, and the mean fecal excretion after 48 hours is 4.9% of the administered dose as metabolites of the parent drug.
It was observed that, after intramuscular administration of 6,00,000 IU vitamin D3 in elderly patients, mean serum calcidiol concentration increases to 32.72±9.0 ng/ml at 6th week and up to 52.34±14.2 ng/ml at 12th week from baseline (11.76±7.6 ng/ml).
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
None stated.
7.0 Description
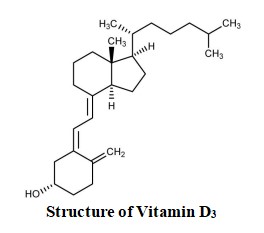
Cholecalciferol is the naturally occurring form of vitamin D, also called vitamin D3. It is produced from 7-dehydrocholesterol, a sterol present in mammalian skin, after being exposed to ultraviolet radiation.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None stated.
8.2 Shelf-life
Refer on pack
8.3 Packaging information
3 ampoules of 1 ml each
8.4 Storage and handing instructions
Store below 25°C. Protect from light. After opening the residual must be discarded. Caution: Do not use if solution is not clear or has suspended matter.
9.0 Patient Counselling Information
- Vitanova-D3 6L injection may interfere with cholesterol tests, hence please inform your physician and laboratory staff that you are taking Vitanova-D3 6L injection before undergoing blood tests.
- Clinical monitoring of serum electrolyte concentrations and cardiac function is recommended.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
-Keep this leaflet. You may need to read it again.
-If you have any further questions, ask your doctor, or pharmacist, or nurse.
-This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
-If you get any side effects, talk to your doctor, or pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4
What is in this leaflet
1.What Vitanova®-D3 6L Injection is and what it is used for
2.What you need to know before you use Vitanova®-D3 6L Injection
3.How to use Vitanova®-D3 6L Injection
4.Possible side effects
5.How to store Vitanova®-D3 6L Injection
6.Contents of the pack and other information
What Vitanova®-D3 6L Injection is and what it is used for
This medicine contains the active ingredient Cholecalciferol Injection which is a form of vitamin D. It belongs to a group of medicines called vitamin D and analogues. Cholecalciferol increases levels of calcium and phosphate in the blood by increasing their absorption from the gut and reducing the amount removed by the kidneys. Cholecalciferol is used for the treatment of severe Vitamin-D deficiency. Few examples are as follows:
-Weak bones and teeth (called ‘Rickets’)
-Low levels of phosphate in the blood
-Other problems with how bones are formed (osteomalacia).
These disorders are due to poor absorption of vitamin D within the body usually caused by diseases of the gut, liver or gall bladder.
It is important that you have this medicine so that your bones and teeth form properly.
1. What you need to know before you use Vitanova®-D3 6L Injection
Do not use Vitanova®-D3 6L Injection:
• if you are allergic to vitamin D3 or any of the other ingredients of this medicine;
• if you have high levels of calcium in your blood (hypercalcaemia) or urine (hypercalciuria);
• if you have kidney stones (renal calculi) or serious kidney problems;
• if you have high levels of vitamin D3 in your blood (Hypervitaminosis D).
• if you have an accumulation of calcium salts in the body's tissue. If any of the above applies to you talk to your doctor or nurse.
Warnings and Precautions
Talk to your doctor or pharmacist or nurse before using Vitanova®-D3 6L Injection if you:
• if you have heart disease, problems with your kidneys or with your circulation
• if you have kidney stones
• if you have low levels of parathyroid hormone (PTH)
• if you already have high levels of vitamin D in your blood or if you are especially sensitive to vitamin D.
It is important that you are taking enough calcium in your diet so that your body can respond properly to your medicine.
Other medicines and Vitanova®-D3 6L Injection
Tell your doctor if you are taking, have recently taken or might take any other medicines.
• Medicines for heart disease such as digoxin or verapamil as these can cause high levels of calcium in the blood leading to an irregular or fast heartbeat.
• Antacids containing magnesium for indigestion. If you are on kidney dialysis this can lead to high levels of magnesium in the blood which causes muscle weakness, low blood pressure, depression and coma.
• Thiazide diuretics (‘water tablets’) to relieve water retention such as bendroflumethiazide as these can lead to high levels of calcium if your body does not produce enough parathyroid hormone (PTH).
• Phenytoin, carbamazepine, primidone or phenobarbital used for the treatment of epilepsy as these may cause Cholecalciferol to be lost from the body too quickly.
• Any other medicine, including medicines obtained without a prescription.
If any of the above applies to you talk to your doctor or nurse before being given Cholecalciferol.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before being given this medicine. Your doctor will tell you if you need to have Cholecalciferol when you are pregnant. You should not be given Cholecalciferol if you are breast-feeding. This medicine will be given to you in pregnancy only if your doctor decides it is necessary.
Driving and using machines
There is limited information on the possible effects of this medicine on your ability to drive. However, it is not expected that it would affect your ability to drive or to operate machinery.
2. How you will be given Cholecalciferol
Cholecalciferol will be given to you by your doctor or nurse. Important: Your doctor will choose the dose that is right for you. You will be given Cholecalciferol by your doctor or nurse as an injection into a muscle. You may be given it just once or it may be repeated depending on how much your body needs. Your doctor may also prescribe for you other medicines containing calcium and phosphorus.
The recommended dose: a single dose of 6,00,000 IU intramuscularly once only or repeated after 6 months to 1 year, depending upon clinical response and requirements.
Medical check-ups
While you are receiving this medicine, your doctor will test your blood and urine regularly. This is to make sure that your medicine is working properly and that the dose you are taking is right for you. If the level of sugar in your blood becomes higher than normal your doctor will stop your treatment with Cholecalciferol.
If you are given more Cholecalciferol than you should
If you think you have been given too much Cholecalciferol you should tell your doctor.
If you have too much Cholecalciferol you may get increased calcium levels in your blood which may lead to the following: feeling sick, feeling weak, weight loss, stiffness, constipation, diarrhoea, hallucinations, fainting, increased levels of urination or feeling thirsty.
If you have any further questions on the use of this medicine, ask your doctor or nurse.
3. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Side effects include:
Very common: may affect more than 1 in 10 people
• Too much calcium in blood (hypercalcaemia)
• Decrease activity of parathyroid gland (hypoparathyroidism)
• Disturbed parathyroid hormone metabolism (pseudohypoparathyroidism)
• Increased levels of creatinine in the blood which may indicate kidney problems.
Rare: may affect up to 1 in 1,000 people
• Psychosis – changes in abilities and personality
Not known: frequency cannot be estimated from the available data
Effects on the heart and blood:
• Hardening of the blood vessels, Irregular heart beat
• High blood pressure, High cholesterol, Imbalanced salt levels.
Effects on liver or kidneys:
• Kidney problems including kidney stones
• Increase in liver enzymes
• Protein in the urine.
Effects on the stomach and bowel:
• Inflammation of the pancreas, which causes severe pain in the abdomen and back
• Feeling sick or being sick
• Constipation, Diarrhoea
• Loss of appetite, Weight loss.
Effects on thirst and urinating:
• Dry mouth, a metallic taste, Drinking a lot
• Passing a lot of urine especially at night.
Effects on the eyes and skin:
• Conjunctivitis, Sensitivity to light, Itchy skin.
Effects on mood
• Being irritable, Loss of sex drive.
Effects on the nervous system:
• Weakness, Headache, Dizziness, Sleepiness.
Other effects:
• Calcium deposits in parts of the body other than bone
• Muscle pain, Bone pain, Tiredness, Feeling too hot, Runny nose.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
4. How to store VITANOVA- D3
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and container after "Exp". The expiry date refers to the last day of that month.
- Store below 25°C. Protect from light.
- After opening the residual must be discarded. Do not use this medicine if you notice the solution is cloudy
- Caution: Do not use if solution is not clear or has suspended matter.
- Keep the container in the outer carton in order to protect from light.
- Do not freeze or refrigerate.
5. Contents of the pack and other information
What Vitanova®-D3 6L Injection contains
Each ml contains:
Cholecalciferol IP 6,00,000 IU (15mg)
Ethyl Oleate IP q.s.
What Vitanova®-D3 6L Injection looks like and contents of pack
Packaging: 3 ampoules of 1 ml each