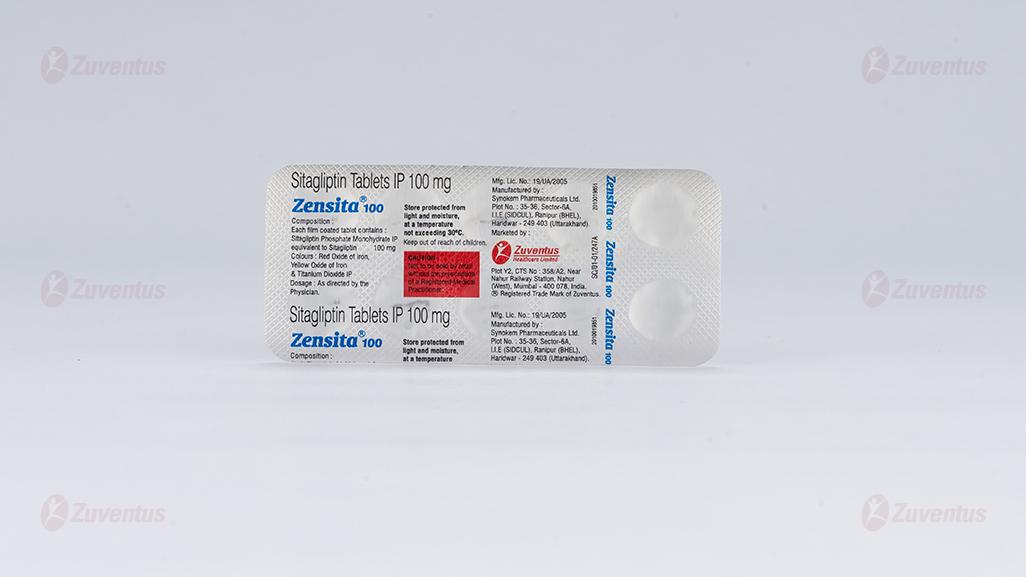
Zensita 100 Tablets
Therapy Area
Anti-diabetic
1.0 Generic name
Sitagliptin Tablets IP 50 mg / 100 mg
2.0 Qualitative and quantitative composition
Zensita 50
Each film coated tablet contains : Sitagliptin Phosphate Monohydrate IP equivalent to Sitagliptin 50 mg Colours : Red Oxide of Iron, Yellow Oxide of Iron & Titanium Dioxide IP
Zensita 100
Each film coated tablet contains : Sitagliptin Phosphate Monohydrate IP equivalent to Sitagliptin 100 mg Colours : Red Oxide of Iron, Yellow Oxide of Iron & Titanium Dioxide IP
3.0 Dosage form and strength
Film coated tablet 50 mg /100 mg
4.0 Clinical particulars
4.1 Therapeutic indication
- As adjunct to diet and exercise to improve glycemic control in patients with type-II diabetes mellitus.
- Use of Sitagliptin Phosphate in combination with Metformin and a PPARy agonist as an adjunct to diet & exercise in adult patients with type-2 Diabetes mellitus who are inadequately controlled on combination therapy with Mefformin and a PPARy agonist.
- Use of Sitagliptin Phosphate in combination with Insulin, alone or in combination with Mefformin.
4.2 Posology and method of administration
The dose is 100 mg Sitagliptin once daily. When used in combination with Metformin and/or a PPARy agonist, the dose of Metformin and/or PPARy agonist should be maintained, and Sitagliptin Phosphate Tablets administered concomitantly. When Sitagliptin Phosphate Tablets is used in combination with a Sulphonylurea or with Insulin, a lower dose of the Sulphonylurea or Insulin may be considered to reduce the risk of hypoglycaemia. If a dose of Sitagliptin Phosphate Tablets is missed, it should be taken as soon as the patient remembers. A double dose should not betaken on the same day. Special population Renal impairment When considering the use of Sitagliptin in combination with another anti-diabetic medicinal product, its conditions for use in patients with renal impairment should be checked. For patients with mild renal impairment (glomerular filtration rate [GFRJ a. 60 to < 90 mUmin), no dose adjustment is required. For patients with moderate renal impairment (GFR 45 to < 60 mUmin), no dosage adjustment is required. For patients with moderate renal impairment (GFR 30 to < 45 mUmin), the dose of Sitagliptin Phosphate Tablets is 50 mg once daily. For patients with severe renal impairment (GFR z 15 to <30 mUmin) or with end-stage renal disease (ESRD) (GFR < 15 mUmin), including those requiring haemodialysis or peritoneal dialysis, the dose of Sitagliptin Phosphate Tablets is 25 mg once daily. Treatment may be administered without regard to the timing of dialysis. Because there is a dosage adjustment based upon renal function, assessment of renal function is recommended prior to initiation of Sitagliptin Phosphate Tablets and periodically thereafter. Hepatic impairment No dose adjustment is necessary for patients with mild to moderate hepatic impairment. Sitagliptin Phosphate Tablets has not been studied in patients with severe hepatic impairment and care should be exercised. However, because Sitagliptin is primarily renally eliminated, severe hepatic impairment is not expected to affect the pharmacokinetics of Sitagliptin. Elderly No dosage adjustment is required. Paediatric population Sitagliptin should not be used in children and adolescents 10 to 17 years of age because of insufficient efficacy. Sitagliptin has not been studied in paediatric patients under 10 years of age. Method of administration For oral use Sitagliptin Phosphate Tablets I.P. can be taken with or without food.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in the formulation.
4.4 Special warnings and precautions for use
General
Zensita should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis. Acute pancreatitis Use of OPP-4 inhibitors has been associated with a risk of developing acute pancreatitis. Patients should be informed of the characteristic symptom of acute pancreatitis : persistent, severe abdominal pain. Resolution of pancreatitis has been observed after discontinuation of Sitagliptin (with or without supportive treatment), but very rare cases of necrotising or haemontagic pancreatitis and/or death have been reported. If pancreatitis is suspected, Zensita and other potentially suspect medicinal products should be discontinued; if acute pancreatitis is confirmed, Zensita should not be restarted. Caution should be exercised in patients with a history of pancreatitis. Hypoglycaemia when used in combination with other anti-hyperglycaemic medicinal products. In clinical trials of ZensIta as monotherapy and as part of combination therapy with medicinal products not known to cause hypoglycaemia (i.e. Metformin and/or a PPARy agonist), rates of hypoglycaemia reported with Sitagliptin were similar to rates in patients taking placebo. Hypoglycaemia has been observed when Sitagliptin was used in combination with Insulin or a Sulphonylurea. Therefore, to reduce the risk of hypoglycaemia, a lower dose of Sulphonylurea or Insulin may be considered. Renal impairment Sitagliptin is renally excreted. To achieve plasma concentrations of Sitagliptin similar to those in patients with normal renal function, lower dosages are recommended in patients with GFR < 45 ra/min, as well as in ESRD patients requiring haemodialysis or peritoneal dialysis. When considering the use of Sitagliptin in combination with another anti-diabetic medicinal product, its conditions for use in patients with renal impairment should be checked. Hypersensitivity reactions Post-marketing reports of serious hypersensitivity reactions in patients treated with Sitagliptin have been reported. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. Onset of these reactions occurred within the first 3 months after initiation of treatment, with some reports occurring after the first dose. If a hypersensitivity reaction is suspected, Zensita should be discontinued. Other potential causes for the event should be assessed, and alternative treatment for diabetes initiated. Bullous pemphigoid There have been post-marketing reports of bullous pemphigoid in patients taking DPP-4 inhibitors including Sitagliptin. If bullous pemphigoid is suspected, Zensita should be discontinued.
4.5 Drugs interactions
Effects of other medicinal products on Sitagliptin
Clinical data described below suggest that the risk for clinicalh/ meaningful interactions by co-administered medicinal products Is low. In vitro studies indicated that the primary enzyme responsible for the limited metabolism of Sitagliptin is CYP3A4, with contribution from CYP2C8. In patients with normal renal function, metabolism, including via CYP3A4, plays only a small role in the clearance of Sitagliptin. Metabolism may play a more significant role in the elimination of Sitagliptin in the setting of severe renal impairment or end-stage renal disease (ESRD). For this reason, it is possible that potent CYP3A4 inhibitors (i.e. Ketoconazole, ftraconazole, Ritonavir, Clarithromycin) could alter the pharmacokinetics of Sitagliptin in patients with severe renal impairment or ESRD. The effect of potent CYP3A4 inhibitors in the setting of renal impairment has not been assessed in a clinical study. In vitro transport studies showed that Sitagliptin is a substrate for p-glycoprotein and organic anion transporter-3 (OAT3). OAT3 mediated transport of Sitagliptin was inhibited in vitro by probenecid, although the risk of clinically meaningful interactions is considered to be low. Concomitant administration of OAT3 inhibitors has not been evaluated in vivo. Mefformin Co-administration of multiple twice-daily doses of 1,000 mg Metformin with 50 mg Sitagliptin did not meaningfully alter the pharmacokinetics of Sitagliptin in patients with type 2 diabetes. Ciclosporin A study was conducted to assess the effect of Ciclosporin, a potent inhibitor of p-glycoprotein, on the pharmacokinetics of Sitagliptin. Co-administration of a single 100 mg oral dose of Sitagliptin and a single 600 mg oral dose of Ciclosporin increased the AUC and Cmax of Sitagliptin by approximately 29% and 68%, respectively. These changes in Sitagliptin pharmacokinetics were not considered to be clinically meaningful. The renal clearance of Sitagliptin was not meaningfully altered. Therefore, meaningful interactions would not be expected with other p-glycoprotein inhibitors. Effects of Sitagliptin on other medicinal products Digoxin Sitagliptin had a small effect on plasma Digoxin concentrations. Following administration of 0.25 mg Digoxin concomitantly with 100 mg of Sitagliptin daily for 10 days, the plasma AUC of digoxin was increased on average by 11%, and the plasma Cmax on average by 18%. No dose adjustment of Digoxin is recommended. However, patients at risk of Digoxin toxicity should be monitored for this when Sitagliptin and Digoxin are administered concomitantly. In vitro data suggest that Sitagliptin does not inhibit nor induce CYP450 isoenzymes. In clinical studies, Sitagliptin did not meaningfully alter the pharmacokinetics of Metformin, Glyburide, Simvastatin, Rosiglitazone, Warfarin, or oral contraceptives, providing in vivo evidence of a low propensity for causing interactions with substrates of CYP3A4, CYP2C8, CYP2C9, and organic cationic transporter (OCT). Sitagliptin may be a mild inhibitor of p-glycoprotein in Wyo.
4.6 Use in special populations
Pregnancy
There are no adequate data from the use of Sitagliptin in pregnant women. Studies in animals have shown reproductive toxicity at high doses. The potential risk for humans is unknown. Oue to lack of human data. Zensita should not be used during pregnancy.
Breast-feeding
It is unknown whether Sitagliptin is excreted in human breast milk. Animal studies have shown excretion of Sitagliptin in breast milk. Zensita should not be used during breast-feeding. Fertility Animal data do not suggest an effect of treatment with Sitagliptin on male and female fertility. Human data are lacking.
4.7 Effects on ability to drive and use machines
Zensita has no or negligible influence on the ability to drive and use machines. However, when driving or using machines, it should be taken into account that dizziness and somnolence have been reported. In addition, patients should be alerted to the risk of hypoglycaemia when Zensita is used in combination with a Sulphonylurea or with Insulin.
4.8 Undesirable effects
Serious adverse reactions including pancreatitis and hypersensitivity reactions have been reported. Hypoglycaemia has been reported in combination with Sulphonylurea and Insulin.
Tabulated list of adverse reactions Adverse reactions are listed below (Table 1) by system organ class and frequency. Frequencies are defined as: very common (a 1/10); common (a 1/100 to < 1/10); uncommon (a 1/1.000 to < 1/100); rare (a 1/10,000 to < 1/1.000); very rare (< 1/10,000) and not known (cannot be estimated from the available data).
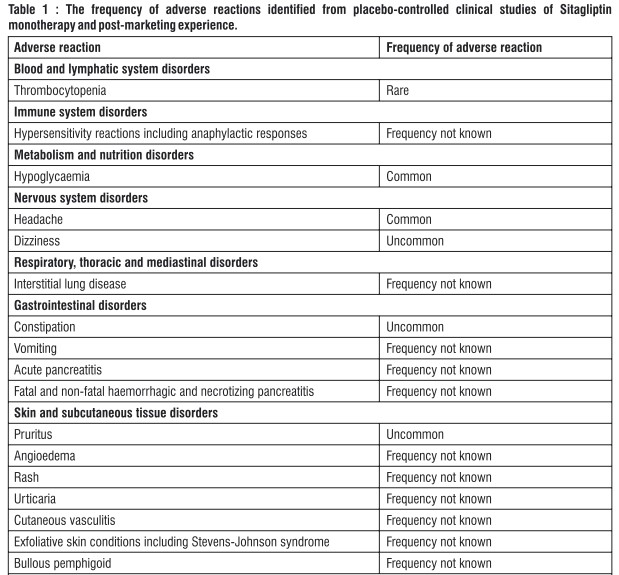
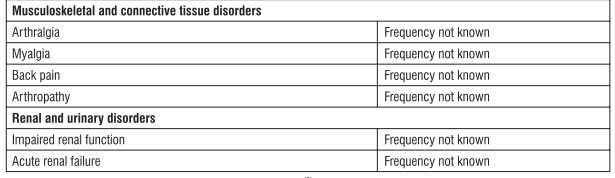
Description of selected adverse reactions In addition to the drug-related adverse experiences described above, adverse experiences reported regardless of causal relationship to medication and occurring in at least 5% and more commonly in patients treated with Sitagliptin included upper respiratory tract infection and nasopharyngitis. Additional adverse experiences reported regardless of causal relationship to medication that occurred more frequently in patients treated with Sitagliptin (not reaching the 5% level, but occurring with an incidence of > 0.5% higher with Sitagliptin than that in the control group) included osteoarthritis and pain in extremity. Some adverse reactions were observed more frequently in studies of combination use of Sitagliptin with other anti-diabetic medicinal products than in studies of Sitagliptin monotherapy. These included hypoglycaemia (frequency very common with the combination of Sulphonylurea and Metformin), influenza (common with Insulin (with or without Metformin)), nausea and vomiting (common with Metformin), flatulence (common with Metformin or Pioglitazone), constipation (common with the combination of Sulphonylurea and Metformin), peripheral oedema (common with Pioglitazone or the combination of Pioglitazone and Metformin), somnolence and diarrhoea (uncommon with Metformin), and dry mouth (uncommon with Insulin (with or without Metformin)). Paediatric population In clinical trials with Sitagliptin in paediatric patients with type 2 diabetes mellitus aged 10 to 17 years, the profile of adverse reactions was comparable to that observed in adults. TECOS cardiovascular safety study The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) included 7,332 patients treated with Sitagliptin, 100 mg daily (or 50 mg daily if the baseline eGFR was ?_ 30 and < 50 mi./min/1.73 m2), and 7,339 patients treated with placebo in the intention-to-treat population. Both treatments were added to usual care targeting regional standards for HbAjc and CV risk factors. The overall incidence of serious adverse events in patients receiving Sitagliptin was similar to that in patients receiving placebo. In the intention-to-treat population, among patients who were using Insulin and/or a Sulfonylurea at baseline, the incidence of severe hypoglycaemia was 2.7%in Sitagliptin-treated patients and 2.5% in placebo-treated patients: among patients who were not using Insulin and/or a Sulfonylurea at baseline, the incidence of severe hypoglycaemia was 1.0% in Sitagliptin-treated patients and 0.7% in placebo-treated patients. The incidence of adjudication-confirmed pancreatitis events was 0.3% in sitagliptin-treated patients and 0.2%in placebo-treated patients. Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com. By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Owing controlled clinical trials in healthy subjects, single doses of up to 800 mg Sitagliptin were administered. Minimal increases in QTc, not considered to be clinically relevant, were observed in one study at a dose of 800 mg Sitagliptin. There is no experience with doses above 800 mg in clinical studies. In Phase I multiple-dose studies, there were no dose-related clinical adverse reactions observed with Sitagliptin with doses of up to 600 mg per day for periods of up to 10 days and 400 mg per day for periods of up to 28 days. In the event of an overdose, it is reasonable to employ the usual supportive measures, e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring (including obtaining an electrocardiogram), and institute supportive therapy if required. Sitagliptin is modestly dialysable. In clinical studies, approximately 13.5% of the dose was removed over a 3 to 4 hour haemodialysis session. Prolonged haemodialysis may be considered if clinically appropriate. It is not known if Sitagliptin is dialysable by peritoneal dialysis.
5.0 Pharmacological properties
5.1 Mechanism of action
Sitagliptin is a DPP-4 inhibitor, which is believed to exert its actions in patients with type 2 diabetes mellitus by slowing the inactivation of incretin hormones. Concentrations of the active Intact hormones are increased by Sitagliptin, thereby increasing and prolonging the action of these hormones. lncretin hormones, including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. These hormones are rapidly inactivated by the enzyme, DPP-4. The incretins are part of an endogenous system involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase Insulin synthesis and release from pancreatic beta cells by intracellular signaling pathways involving cyclic AMP. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, leading to reduced hepatic glucose production. By increasing and prolonging active incretin levels, Sitagliptin increases Insulin release and decreases glucagon levels in the circulation in a glucose-dependent manner. Sitagliptin demonstrates selectivity for DPP-4 and does not inhibit DPP-8 or DPP-9 activity in vitro at concentrations approximating those from therapeutic doses.
5.2 Pharmacodynamic properties
In patients with type 2 diabetes mellitus, administration of Sitagliptin led to inhibition of DPP-4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP-4 inhibition resulted in a 2 to 3-fold increase in circulating levels of active GLP-1 and GIP decreased glucagon concentrations, and increased responsiveness of Insulin release to glucose, resulting in higher C-peptide and Insulin concentrations. The rise in Insulin with the decrease in glucagon was associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal. In studies with healthy subjects, Sitagliptin did not lower blood glucose or cause hypoglycemia. Sitagliptin and Metformin Hydrochloride co-administration in a two-day study in healthy subjects, Sitagliptin alone increased active GLP-1 concentrations, whereas Metformin alone increased active and total GLP-1 concentrations to similar extents. Co administration of Sitagliptin and Metformin had an additive effect on active GLP-1 concentrations. Sitagliptin, but not Metformin, increased active GIP concentrations. It is unclear how these findings relate to changes in glycemic control in patients with type 2 diabetes mellitus. Cardiac Electrophysiology in a randomized, placebo-controlled crossover study, 79 healthy subjects were administered a single oral dose of Sitagliptin 100 mg, Sitagliptin 800 mg (8 times the recommended dose), and placebo. At the recommended dose of 100 mg, there was no effect on the QTc interval obtained at the peak plasma concentration, or at any other time during the study. Following the 800 mg dose, the maximum increase in the placebo-corrected mean change in OTc from baseline was observed at 3 hours postdose and was 8.0 msec. This increase is not considered to be clinically significant. At the 800 mg dose, peak Sitagliptin plasma concentrations were approximately 11 times higher than the peak concentrations following a 100 mg dose. In patients with type 2 diabetes mellitus administered Sitagliptin 100 mg (N = 81) or Sitagliptin 200 mg (N = 63) daily, there were no meaningful changes in QTc interval based on ECG data obtained at the time of expected peak plasma concentration.
5.3 Pharmacokinetic properties
Absorption
Following oral administration of a 100 mg dose to healthy subjects, Sitagliptin was rapidly absorbed, with peak plasma concentrations (median Imp) occurring 1 to 4 hours post-dose, mean plasma AUC of Sitagliptin was 8.52 pM•tir, Cmax was 950 nM. The absolute bioavailability of Sitagliptin is approximately 87 %. Since co-administration of a high-fat meal with Sitagliptin had no effect on the pharmacokinetics, Zensita may be administered with or without food. Plasma AUC of Sitagliptin increased in a dose-proportional manner. Dose-proportionality was not established for Cmay and CM( (Cmax increased in a greater than dose-proportional manner and C24hr increased in a less than dose-proportional manner).
Distribution
The mean volume of distribution at steady state following a single 100 mg intravenous dose of Sitagliptin to healthy subjects is approximately 198 litres. The fraction of Sitagliptin reversibly bound to plasma proteins is low (38%).
Biotransformation
Sitagliptin is primarily eliminated unchanged in urine, and metabolism is a minor pathway. Approximately 79% of Sitagliptin is excreted unchanged in the urine. Following a [14C] Sitagliptin oral dose, approximately 16% of the radioactivity was excreted as metabolites of Sitagliptin. Six metabolites were detected at trace levels and are not expected to contribute to the plasma DPP-4 Inhibitory activity of Sitagliptin. In vitro studies indicated that the primary enzyme responsible for the limited metabolism of Sitagliptin was CYP3A4, with contribution from CYP2C8. In vitro data showed that Sitagliptin is not an inhibitor of CYP isozymes CYP3A4, 2C8, 2C9, 206, 1A2, 2C19 or 286, and is not an inducer of CYP3A4 and CYP1A2.
Elimination
Following administration of an oral (14C) Sitagliptin dose to healthy subjects, approximately 100% of the administered radioactivity was eliminated in faeces (13%) or urine (87%) within one week of dosing. The apparent terminal tv2 following a 100 mg oral dose of Sitagliptin was approximately 12.4 hours. Sitagliptin accumulates only minimally with multiple doses. The renal clearance was approximately 350 mUmin. Elimination of Sitagliptin occurs primarily via renal excretion and involves active tubular secretion. Sitagliptin is a substrate for human organic anion transporter-3 (hOAT-3), which may be involved in the renal elimination of Sitagliptin. The clinical relevance of hOAT-3 in Sitagliptin transport has not been established. Sitagliptin is also a substrate of p-glycoprotein, which may also be involved in mediating the renal elimination of Sitagliptin. However, Ciclosporin, a p-glycoprotein inhibitor, did not reduce the renal clearance of Sitagliptin. Sitagliptin is not a substrate for OCT2 or OAT1 or PEPT1/2 transporters. In vitro, Sitagliptin did not inhibit OAT3 (IC50 = 160 pM) or p-gtycoprotein (up to 250 pM) mediated transport at therapeutically relevant plasma concentrations. In a clinical study Sitagliptin had a small effect on plasma digoxin concentrations indicating that Sitagliptin may be a mild inhibitor of p-glycoprotein.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Renal and liver toxicity were observed in rodents at systemic exposure values 58 times the human exposure level, while the no-effect level was found at 19 times the human exposure level. Incisor teeth abnormalities were observed in rats at exposure levels 67 times the clinical exposure level; the no-effect level for this finding was 58-fold based on the 14-week rat study. The relevance of these findings for humans is unknown. Transient treatment-related physical signs, some of which suggest neural toxicity, such as open-mouth breathing, salivation, white foamy emesis, ataxia, trembling, decreased activity, and/or hunched posture were observed in dogs at exposure levels approximately 23 times the clinical exposure level. In addition, very slight to slight skeletal muscle degeneration was also observed histologically at doses resulting in systemic exposure levels of approximately 23 times the human exposure level. A no-effect level for these findings was found at an exposure 6-fold the clinical exposure level. Sitagliptin has not been demonstrated to be genotoxic in preclinical studies. Sitagliptin was not carcinogenic in mice. In rats, there was an increased Incidence of hepatic adenomas and carcinomas at systemic exposure levels 58 times the human exposure level. Since hepatotoxicity has been shown to correlate with induction of hepatic neoplasia in rats, this increased incidence of hepatic tumours in rats was likely secondary to chronic hepatic toxicity at this high dose. Because of the high safety margin (19-fold at this no-effect level), these neoplastic changes are not considered relevant for the situation in humans. No adverse effects upon fertility were observed in male and female rats given Sitagliptin prior to and throughout mating. In a pre-/postnatal development study performed in rats Sitagliptin showed no adverse effects. Reproductive toxicity studies showed a slight treatment-related increased incidence of foetal rib malformations (absent, hypoplastic and wavy ribs) in the offspring of rats at systemic exposure levels more than 29 times the human exposure levels. Maternal toxicity was seen in rabbits at more than 29 times the human exposure levels. Because of the high safety margins, these findings do not suggest a relevant risk for human reproduction. Sitagliptin is secreted in considerable amounts into the milk of lactating rats (milk/plasma ratio :4:1).
7.0 Description
Zensita Tablets contain Sitagliptin Phosphate, an orally-active inhibitor of the dipeptidyl peptidase 4 (DPP-4) enzyme. Sitagliptin Phosphate Monohydrate is described chemically as 7-1(3R)-3-amino-1-oxo-4-(2,4,5-Mfluorophenyl)butyll-5.6,7.8-tetrahydro-3-(trifluoromethyl)-1,2,4-trlazolo [4,3- a)pyrazine phosphate (1:1) monohydrate.
Empirical formula : C16H15F6N50•H3PO4• H20
Molecular weight : 523.32 g/mol
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shell-life
Refer on the pack.
8.3 Packaging information
PVC/PVDC-Alu blister strip of 10 tablets.
8.4 Storage and handing instructions
Store protected from light and moisture, at a temperature not exceeding 30°C.
Keep out of reach of children.
9.0 Patient counselling information
Pancreatitis
Inform patients that acute pancreatitis has been reported during post marketing use of Zensita. Inform patients that persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to promptly discontinue Zensita and contact their physician if persistent severe abdominal pain occurs.
Heart failure
Inform patients of the signs and symptoms of heart failure. Before initiating Zensita, ask patients about a history of heart failure or other risk factors for heart failure including moderate to severe renal impairment. Instruct patients to contact their health care provider as soon as possible if they experience symptoms of heart failure, including increasing shortness of breath, rapid increase in weight or swelling of the feet.
Hypoglycemia
Inform patients that the incidence of hypoglycemia is increased when Zensita is added to a Sulfonylurea or Insulin. Explain to patients receiving Zensita in combination with these medications the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development.
Hypersensitivity reactions
Inform patients that allergic reactions have been reported during post-marketing use of Zensita. If symptoms of allergic reactions (including rash, hives, and swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing) occur, patients must stop taking Zensita and seek medical advice promptly.
Severe and disabling arthralgia
Inform patients that severe and disabling joint pain may occur with this class of drugs. The time to onset of symptoms can range from one day to years. Instruct patients to seek medical advice if severe joint pain occurs.
Bullous pemphigoid
Inform patients that bullous pemphigoid may occur with this class of drugs. Instruct patients to seek medical advice if blisters or erosions occur.
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Sitagliptin is and what it is used for
2. What you need to know before you take Sitagliptin
3. How to take Sitagliptin
4. Possible side effects
5. How to store Sitagliptin
6. Contents of the pack and other information
1. What Sitagliptin is and what it is used for
Sitagliptin contains the active substance sitagliptin which is a member of a class of medicines called DPP-4 inhibitors (dipeptidyl peptidase-4 inhibitors) that lowers blood sugar levels in adult patients with type 2 diabetes mellitus. This medicine helps to increase the levels of insulin produced after a meal and decreases the amount of sugar made by the body. Your doctor has prescribed this medicine to help lower your blood sugar, which is too high because of your type 2 diabetes. This medicine can be used alone or in combination with certain other medicines (insulin, metformin, sulphonylureas, or glitazones) that lower blood sugar, which you may already be taking for your diabetes together with a food and exercise plan.
What is type 2 diabetes?
Type 2 diabetes is a condition in which your body does not make enough insulin, and the insulin that your body produces does not work as well as it should. Your body can also make too much sugar. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems like heart disease, kidney disease, blindness, and amputation.
2. What you need to know before you take Sitagliptin
Do not take Sitagliptin
- if you are allergic to sitagliptin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Cases of inflammation of the pancreas (pancreatitis) have been reported in patients receiving Sitagliptin (see section 4).
If you encounter blistering of the skin it may be a sign for a condition called bullous pemphigoid.
Your doctor may ask you to stop Sitagliptin.
Tell your doctor if you have or have had:
a disease of the pancreas (such as pancreatitis)
gallstones, alcohol dependence or very high levels of triglycerides (a form of fat) in your blood. These medical conditions can increase your chance of getting pancreatitis (see section 4).
- type 1 diabetes
- diabetic ketoacidosis (a complication of diabetes with high blood sugar, rapid weight loss, nausea or vomiting)
- any past or present kidney problems.
- an allergic reaction to Sitagliptin (see section 4).
This medicine is unlikely to cause low blood sugar because it does not work when your blood sugar is low. However, when this medicine is used in combination with a sulphonylurea medicine or with insulin, low blood sugar (hypoglycaemia) can occur. Your doctor may reduce the dose of your sulphonylurea or insulin medicine.
Children and adolescents
Children and adolescents below 18 years should not use this medicine. It is not effective in children and adolescents between the ages of 10 and 17 years. It is not known if this medicine is safe and effective when used in children under 10 years.
Other medicines and Sitagliptin
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
In particular, tell your doctor if you are taking digoxin (a medicine used to treat irregular heart beat and other heart problems). The level of digoxin in your blood may need to be checked if taking with Sitagliptin.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. You should not take this medicine during pregnancy. It is not known if this medicine passes into breast milk. You should not take this medicine if you are breast-feeding or plan to breast-feed.
Driving and using machines
This medicine has no or negligible influence on the ability to drive and use machines. However, dizziness and drowsiness have been reported, which may affect your ability to drive or use machines.
Taking this medicine in combination with medicines called sulphonylureas or with insulin can cause hypoglycaemia, which may affect your ability to drive and use machines or work without safe foothold.
Sitagliptin contains Sodium
This medicine contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
3. How to take Sitagliptin
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The usual recommended dose is:
- one 100 mg film-coated tablet
- once a day
- by mouth
If you have kidney problems, your doctor may prescribe lower doses (such as 25 mg or 50 mg). You can take this medicine with or without food and drink. Swallow the tablets whole; do not crush or chew. Your doctor may prescribe this medicine alone or with certain other medicines that lower blood sugar.
Diet and exercise can help your body use its blood sugar better. It is important to stay on the diet and exercise recommended by your doctor while taking Sitagliptin.
If you take more Sitagliptin than you should
If you take more than the prescribed dosage of this medicine, contact your doctor immediately.
If you forget to take Sitagliptin
If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule.
Do not take a double dose of this medicine.
If you stop taking Sitagliptin Continue to take this medicine as long as your doctor prescribes it so you can continue to help control your blood sugar. You should not stop taking this medicine without talking to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. STOP taking Sitagliptin and contact a doctor immediately if you notice any of the following serious side effects:
- Severe and persistent pain in the abdomen (stomach area) which might reach through to your back with or without nausea and vomiting, as these could be signs of an inflamed pancreas (pancreatitis).
If you have a serious allergic reaction (frequency not known), including rash, hives, blisters on the skin/peeling skin and swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing, stop taking this medicine and call your doctor right away. Your doctor may prescribe a medicine to treat your allergic reaction and a different medicine for your diabetes.
Some patients have experienced the following side effects after adding sitagliptin to metformin:
Common (may affect up to 1 in 10 people):
- low blood sugar,
- nausea,
- flatulence,
- vomiting. Uncommon (may affect up to 1 in 100 people):
- stomach ache,
- diarrhoea,
- constipation,
- drowsiness.
Some patients have experienced different types of stomach discomfort when starting the combination of sitagliptin and metformin together (frequency is common).
Some patients have experienced the following side effects while taking sitagliptin in combination with a sulphonylurea and metformin:
Very common (may affect more than 1 in 10 people):
- low blood sugar. Common:
- constipation. Some patients have experienced the following side effects while taking sitagliptin and pioglitazone: Common:
- flatulence,
- swelling of the hands or legs.
Some patients have experienced the following side effects while taking sitagliptin in combination with pioglitazone and metformin:
Common:
- swelling of the hands or legs.
- Some patients have experienced the following side effects while taking sitagliptin in combination with insulin (with or without metformin):
- Common:
- flu. Uncommon:
- dry mouth.
Some patients have experienced the following side effects while taking sitagliptin alone in clinical studies, or during post-approval use alone and/or with other diabetes medicines:
Common:
- low blood sugar,
- headache,
- upper respiratory infection,
- stuffy or runny nose and sore throat,
- osteoarthritis,
- arm or leg pain.
Uncommon:
- dizziness,
- constipation,
- itching. Rare:
- reduced number of platelets Frequency not known:
- kidney problems (sometimes requiring dialysis),
- vomiting,
- joint pain,
- muscle pain,
- back pain,
- interstitial lung disease,
- bullous pemphigoid (a type of skin blister).
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Sitagliptin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister or bottle label and the carton after ‘EXP’. The expiry date refers to the last day of that month.
Shelf life after first opening of the HDPE container: 1 month.
This medicine does not require any special storage conditions.
Do not throw away medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Sitagliptin contains
The active substance is sitagliptin.
Each film-coated tablet contains 50 mg sitagliptin (as hydrochloride).
Each film-coated tablet contains 100 mg sitagliptin (as hydrochloride).
Zensita is available in the following pack sizes:
PVC/PVDC-Alu blister strip of 10 tablets.