Zensita Trio 1000 Tablets
Therapy Area
Anti-diabetic
1.0 Generic name
Dapagliflozin, Sitagliptin and Metformin Hydrochloride (as extended release) Tablets (10 mg + 100 mg + 500 mg)
Dapagliflozin, Sitagliptin and Metformin Hydrochloride (as extended release) Tablets (10 mg + 100 mg + 1000 mg)
2.0 Qualitative and quantitative composition
Zensita-Trio 500
Each film coated tablet contains :
Dapagliflozin Propanediol Monohydrate
equivalent to Dapagliflozin 10 mg
Sitagliptin Phosphate Monohydrate IP
equivalent to Sitagliptin 100 mg
Metformin Hydrochloride IP 500 mg
(as extended release)
Excipients q.s.
Colours : Ferric Oxide Red USP-NF, Ferric
Oxide Yellow USP-NF & Titanium Dioxide IP
Zensita-Trio 1000
Each fllm coated tablet contains :
Dapagliflozin Propanediol Monohydrate
equivalent to Dapagliflozin 10 mg
Sitagliptin Phosphate Monohydrate IP
equivalent to Sitagliptin 100 mg
Metformin Hydrochloride IP 1000 mg
(as extended release)
Excipients q.s.
Colours : Indigo Carmine Aluminium Lake & Titanium Dioxide IP
3.0 Dosage form and strength
Film coated tablet, Dapagliflozin 10 mg + Sitagliptin 100 mg + Metformin Hydrochloride Extended Release 500 mg/ 1000 mg tablets
4.0 Clinical particulars
4.1 Therapeutic indication
It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
4.2 Posology & method of administration
Posology
The recommended dose is one tablet daily.
Elderly (≥ 65 years)
Because metformin is eliminated in part by the kidney, and because elderly patients are more likely to have decreased renal function, this medicinal product should be used with caution as age increases.
Paediatric population
The safety and efficacy of Dapagliflozin, Sitagliptin and Metformin Hydrochloride ER tablets have not yet been established. No data are available.
Method of administration
Dapagliflozin, Sitagliptin and Metformin Hydrochloride ER tablets should be given once daily with meals to reduce the gastrointestinal adverse reactions associated with metformin. It should be given orally once daily.
4.3 Contraindications
• Hypersensitivity to the active substances or to any sodium-glucose co-transporter 2 (SGLT2) inhibitor or to any dipeptidyl peptidase-4 (DPP-4) inhibitor or to any of the excipients listed in the formulation.
• Any type of acute metabolic acidosis (such as lactic acidosis, diabetic ketoacidosis).
• Diabetic pre-coma.
• Moderate 2 to severe renal impairment (eGFR less than 45 mL/min/1.73 m ), end-stage renal disease (ESRD), or patients on dialysis.
• Acute conditions with the potential to alter renal function such as: dehydration, severe infection, shock.
• Disease which may cause tissue hypoxia (especially acute disease, or worsening of chronic disease) such as : decompensated heart failure, respiratory failure, recent myocardial infarction, shock.
• Hepatic insufficiency, acute alcohol intoxication, alcoholism.
4.4 Warning & precautions
Renal impairment
Zensita-Trio is contraindicated in patients with moderate to severe renal impairment 2 (eGFR less than 45 mL/min/1.73 m ), ESRD, or on dialysis.
Use in patients at risk for volume depletion and/or hypotension Dapagliflozin increases diuresis which may lead to the modest decrease in blood pressure observed in clinical studies. It may be more pronounced in patients with very high blood glucose concentrations. Caution should be exercised in patients for whom a dapagliflozin-induced drop in blood pressure could pose a risk, such as patients on anti-hypertensive therapy with a history of hypotension or elderly patients.
In case of intercurrent conditions that may lead to volume depletion (e.g. gastrointestinal illness), careful monitoring of volume status (e.g. physical examination, blood pressure measurements, laboratory tests including hematocrit and electrolytes) is recommended. Temporary interruption of treatment with Zensita-Trio is recommended for patients who develop volume depletion until the depletion is corrected.
Diabetic ketoacidosis
Rare cases of diabetic ketoacidosis (DKA), including life-threatening and fatal cases, have been reported in patients treated with sodium-glucose co-transporter 2 (SGLT2) inhibitors, including dapaglifozin. In a number of cases, the presentation of the condition was atypical with only moderately increased blood glucose values, below 14 mmol/L (250 mg/dL).
The risk of diabetic ketoacidosis must be considered in the event of non-specific symptoms such as nausea, vomiting, anorexia, abdominal pain, excessive thirst, difficulty breathing, confusion, unusual fatigue or sleepiness. Patients should be assessed for ketoacidosis immediately if these symptoms occur, regardless of blood glucose level.
In patients where DKA is suspected or diagnosed, Zensita-Trio treatment should be stopped immediately.
Treatment should be interrupted in patients who are hospitalized for major surgical procedures or acute serious medical illnesses. Monitoring of ketones is recommended in these patients. Measurement of blood ketone levels is preferred to urine. Treatment with Zensita-Trio may be restarted when the ketone values are normal and the patient's condition has stabilized.
Before initiating Zensita-Trio, factors in the patient history that may predispose to ketoacidosis should be considered. Patients who may be at higher risk of DKA include patients with a low beta-cell function reserve (e.g. type 2 diabetes patients with low C-peptide or latent autoimmune diabetes in adults (LADA) or patients with a history of pancreatitis), patients with conditions that lead to restricted food intake or severe dehydration, patients for whom insulin doses are reduced and patients with increased insulin requirements due to acute medical illness, surgery or alcohol abuse. SGLT2 inhibitors should be used with caution in these patients.
Restarting SGLT2 inhibitor treatment in patients experiencing a DKA while on SGLT2 inhibitor treatment is not recommended, unless another clear precipitating factor is identified and resolved.
In type 1 diabetes mellitus studies with dapagliflozin, DKA was reported with common frequency. Zensita-Trio should not be used for treatment of patients with type 1 diabetes
Type 2 diabetes mellitus Rare cases of DKA, including life-threatening and fatal cases, have been reported in patients treated with SGLT2 inhibitors, including dapaglifiozin. In a number of cases, the presentation of the condition was atypical with only moderately increased blood glucose values, below 14 mmol/L (250 mg/dL).
In patients where DKA is suspected or diagnosed, Zensita-Trio treatment should be stopped immediately.
Restarting SGLT2 inhibitor treatment in patients experiencing a DKA while on SGLT2 inhibitor treatment is not recommended, unless another clear precipitating factor is identified and resolved.
Necrotising fasciitis of the perineum (Fournier's gangrene)
Post marketing cases of necrotising fasciitis of the perineum (also known as Fournier's gangrene) have been reported in female and male patients taking SGLT2 inhibitors. This is a rare but serious and potentially life-threatening event that requires urgent surgical intervention and antibiotic treatment. Patients should be advised to seek medical attention if they experience a combination of symptoms of pain, tenderness, erythema, or swelling in the genital or perineal area, with fever or malaise. Be aware that either uro-genital infection or perineal abscess may precede necrotising fasciitis. If Fournier's gangrene is suspected, Zensita-Trio should be discontinued and prompt treatment (including antibiotics and surgical debridement) should be instituted.
Urinary tract infections
Urinary glucose excretion may be associated with an increased risk of urinary tract infection; therefore, temporary interruption of Zensita-Trio should be considered when treating pyelonephritis or urosepsis.
Elderly (≥ 65 years)
Elderly patients may be at a greater risk for volume depletion and are more likely to be treated with diuretics. Elderly patients are more likely to have impaired renal function, and/or to be treated with anti-hypertensive medicinal products that may cause changes in renal function such as angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin II type 1 receptor blockers (ARB). The same recommendations for renal function apply to elderly patients as to all patients.
Lower limb amputations
It is important to counsel patients with diabetes on routine preventative foot care.
Acute pancreatitis
Use of DPP-4 inhibitors has been associated with a risk of developing acute pancreatitis. Patients should be informed of the characteristic symptom of acute pancreatitis: persistent, severe abdominal pain. Resolution of pancreatitis has been observed after discontinuation of sitagliptin (with or without supportive treatment), but very rare cases of necrotising or haemorrhagic pancreatitis and/or death have been reported. If pancreatitis is suspected, Zensita-Trio should be discontinued; if acute pancreatitis is confirmed, Zensita-Trio should not be restarted. Caution should be exercised in patients with a history of pancreatitis. Hypoglycaemia when used in combination with other anti-hyperglycaemic medicinal products Hypoglycaemia has been observed when sitagliptin was used in combination with insulin or a sulphonylurea. Therefore, to reduce the risk of hypoglycaemia, a lower dose of Zensita-Trio or insulin may be considered.
Hypersensitivity reactions
Post-marketing reports of serious hypersensitivity reactions in patients treated with sitagliptin have been reported. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. Onset of these reactions occurred within the first 3 months after initiation of treatment, with some reports occurring after the first dose. If a hypersensitivity reaction is suspected, Zensita-Trio should be discontinued. Other potential causes for the event should be assessed, and alternative treatment for diabetes initiated.
Bullous pemphigoid
There have been post-marketing reports of bullous pemphigoid in patients taking DPP-4 inhibitors including sitagliptin. If bullous pemphigoid is suspected, Zensita-Trio should be discontinued.
Severe and Disabling Arthralgia
There have been post marketing reports of severe and disabling arthralgia in patients taking DPP-4 inhibitors. The time to onset of symptoms following initiation of drug therapy varied from one day to years. Patients experienced relief of symptoms upon discontinuation of the medication. A subset of patients experienced a recurrence of symptoms when restarting the same drug or a different DPP-4 inhibitor. Consider Zensita-Trio as a possible cause for severe joint pain and discontinue drug if appropriate.
Cardiac function
Patients with heart failure are more at risk of hypoxia and renal insufficiency. In patients with stable chronic heart failure, metformin may be used with a regular monitoring of cardiac and renal function. For patients with acute and unstable heart failure, Zensita-Trio is contraindicated.
Surgery
Zensita-Trio must be discontinued at the time of surgery under general, spinal or epidural anesthesia. Therapy may be restarted no earlier than 48 hours following surgery or resumption of oral nutrition and provided that renal function has been reevaluated and found to be stable.
Other precautions
All patients should continue their diet with a regular distribution of carbohydrate intake during the day. Overweight patients should continue their energy-restricted diet. The usual laboratory tests for diabetes monitoring should be performed regularly. Metformin may reduce vitamin B12 serum levels. The risk of low vitamin B12 levels increases with increasing metformin dose, treatment duration, and/or in patients with risk factors known to cause vitamin B12 deficiency. In case of suspicion of vitamin B12 deficiency (such as anemia or neuropathy), vitamin B12 serum levels should be monitored. Periodic vitamin B12 monitoring could be necessary in patients with risk factors for vitamin B12 deficiency. Zensita-Trio therapy should be continued for as long as it is tolerated and not contra-indicated and appropriate corrective treatment for vitamin B12 deficiency provided in line with current clinical guidelines. Metformin alone does not cause hypoglycemia, but caution is advised when it is used in combination with insulin or other oral antidiabetics (e.g. sulfonylureas or meglitinides).
4.5 Drug interactions
Drug Interaction between Dapaglifiozin and Sitagliptin and Glimepiride was evaluated in an open-label, randomized, five-period, five-treatment, unbalanced crossover study. In this study 18 subjects first received 20 mg dapaglifiozin, 4 mg glimepiride or the combination, and afterward 100 mg sitagliptin or sitagliptin plus 20 mg dapaglifiozin. Co-administration of dapaglifiozin with pioglitazone, metformin, glimepiride or sitagliptin had no effect on dapaglifiozin maximum plasma concentration (Cmax) or area under the plasma concentration-time curve (AUC). Similarly, dapaglifiozin did not affect the Cmax or AUC for the co-administered drug, except for slight extensions of the 90% CI for the ratio of geometric means for glimepiride AUC (upper limit 1.29) and pioglitazone Cmax (lower limit 0.75). All monotherapies and combination therapies were well tolerated.
Co-administration of sitagliptin (50 mg twice daily) and metformin (1000 mg twice daily), co-administration of multiple doses of sitagliptin (50 mg twice daily) and metformin (1000 mg twice daily) did not meaningfully alter the pharmacokinetics of either sitagliptin or metformin in patients with type 2 diabetes.
4.6 Special populations
Pregnancy
When pregnancy is detected, treatment with FDC of dapagliflozin, sitagliptin and metformin should be discontinued.
Breastfeeding
FDC of dapaglifozin, sitagliptin and metformin should not be used during breastfeeding.
4.7 Effects on ability to drive and use machines
FDC of Dapagliflozin, sitagliptin, and metformin has no or negligible influence on the ability to drive and use machines. However, when driving or using machines, it should be taken into account that dizziness and somnolence have been reported with sitagliptin.
4.8 Undesirable effects
Metabolism and nutrition disorders
Very common : Hypoglycaemia
Common: Vitamin B12 decrease/deficiency
Uncommon: Volume depletion, thirst
Rare: Diabetic ketoacidosis (when used in type 2 diabetes mellitus)
Very rare: Lactic acidosis.
Nervous system disorders
Common: Taste disturbance, dizziness
Gastrointestinal disorders
Very common: Gastrointestinal disorders such as nausea, vomiting, diarrhoea, abdominal pain and loss of appetite. These undesirable effects occur most frequently during initiation of therapy and resolve spontaneously in most cases. Uncommon: Constipation, dry mouth
Hepatobiliary disorders
Very rare: Isolated reports of liver function tests abnormalities or hepatitis resolving upon metformin discontinuation.
Skin and subcutaneous tissue disorders
Common: Rash
Very rare: Skin reactions such as erythema, pruritus, urticaria, angioedema
Infections and infestations
Common: Vulvovaginitis, balanitis and related genital infections, urinary tract infection
Uncommon: Fungal infection
Very rare: Necrotising fasciitis of the perineum (Fournier's gangrene)
Musculoskeletal and connective tissue disorders
Common: Back pain
Renal and urinary disorders
Common: Dysuria, polyuria
Uncommon: Nocturia
Reproductive system and breast disorders
Uncommon: Vulvovaginal pruritus, pruritus of genital
Investigations
Common : Hematocrit increased, creatinine renal clearance decreased during initial treatment, dyslipidemia
Uncommon : Blood creatinine increased during initial treatment, Blood urea increased, weight decreased
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
Website : https://www.zuventus.com/drug-safety-reporting By reporting side effects, you can help provide more information on the safety of this medicine
4.9 Overdose
In overdose, the stomach should be emptied by aspiration and gastric lavage. Vital parameters should be managed symptomatically and institute supportive measures as required.
5.0 Pharmacological properties
5.1 Mechanism of action
Dapaglifiozin, Sitagliptin and Metformin Hydrochloride ER tablets combines three antihyperglycaemic medicinal products with different and complementary mechanisms of action to improve glycaemic control in patients with type 2 diabetes: (1) Dapaglifiozin, a SGLT2 inhibitor, (2) Sitagliptin, a dipeptidyl peptidase-4 (DPP4) inhibitor, and (3) metformin hydrochloride, a member of the biguanide class.
Sitagliptin
Sitagliptin is a DPP-4 inhibitor, which is believed to exert its actions in patients with type 2 diabetes by slowing the inactivation of incretin hormones. Concentrations of the active
intact hormones are increased by Sitagliptin, thereby increasing and prolonging the action of these hormones. Incretin hormones, including glucagon-like peptide-1 (GLP1) and glucose-dependent insulinotropic polypeptide (GIP), are released by the intestine throughout the day, and levels are increased in response to a meal. These hormones are rapidly inactivated by the enzyme, DPP-4. The incretins are part of an endogenous system involved in the physiologic regulation of glucose homeostasis. When blood glucose concentrations are normal or elevated, GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells by intracellular signaling pathways involving cyclic AMP. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, leading to reduced hepatic glucose production. By increasing and prolonging active incretin levels, Sitagliptin increases insulin release and decreases glucagon levels in the circulation in a glucose-dependent manner. Sitagliptin demonstrates selectivity for DPP4 and does not inhibit DPP-8 or DPP-9 activity in vitro at concentrations approximating those from therapeutic doses.
Dapagliflozin
Sodium-glucose cotransporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Dapagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, dapagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose and thereby increases urinary glucose excretion.
Metformin Hcl
Metformin is an ant hyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral ant hyperglycemic agents. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike sulfonylureas, metformin does not produce hypoglycemia in either patients with type 2 diabetes or normal subjects and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
5.2 Pharmacodynamic properties
Sitagliptin
In patients with type 2 diabetes, administration of sitagliptin led to inhibition of DPP-4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP-4 inhibition resulted in a 2-to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased responsiveness of insulin release to glucose, resulting in higher C-peptide and insulin concentrations. The rise in insulin with the decrease in glucagon was associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Dapagliflozin
Increases in the amount of glucose excreted in the urine were observed in healthy subjects and in subjects with type 2diabetes mellitus following the administration of dapagliflozin.
Metformin
In humans, independently of its action on glycaemia, metformin has favourable effects on lipid metabolism. This has been shown at therapeutic doses in controlled, mediumterm or long-term clinical studies: metformin reduces total cholesterol, LDL cholesterol and triglyceride levels.
5.3 Pharmacokinetic properties
| Dapagliflozin | Sitagliptin | Metformin HCL | |
| Cmax | 158 ng/mL | 491.7 ng/mL | 651.41 ng/mL |
| Tmax | 2 hours | 1-4 hours | 2.5 hours |
| Bioavailability | 78% | 87% | 50-60% |
| T1/2 | 12.9 hours | 12.4 hours | 6.5 hours |
| Volume of distribution (V d) | 118 litres | 198 litres | 63-276 litres |
| Elimination | Dapagliflozin and related metabolites are primarily eliminated via urinary excretion with less than 2% as unchanged dapagliflozin. | Approximately 79 % of sitagliptin is excreted unchanged in the urine and metabolism is a minor pathway | Excreted unchanged in the urine. No metabolites have been identified in humans. |
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Dapagliflozin
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential and fertility. Dapagliflozin did not induce tumors in either mice or rats at any of the doses evaluated in two-year carcinogenicity studies.
Direct administration of dapagliflozin to weanling juvenile rats and indirect exposure during late pregnancy (time periods corresponding to the second and third trimesters of pregnancy with respect to human renal maturation) and lactation are each associated with increased incidence and/or severity of renal pelvic and tubular dilatations in progeny. Therefore, the use of dapagliflozin is not recommended during the second and third trimesters of pregnancy
In a juvenile toxicity study, when dapagliflozin was dosed directly to young rats from postnatal day 21 until postnatal day 90, renal pelvic and tubular dilatations were reported at all dose levels; pup exposures at the lowest dose tested were ≥15 times the maximum recommended human dose (MRHD). These findings were associated with dose-related increases in kidney weight and macroscopic kidney enlargement observed at all doses. The renal pelvic and tubular dilatations observed in juvenile animals did not fully reverse within the approximate 1-month recovery period. In a separate study of pre- and post-natal development, maternal rats were dosed from gestation Day 6 through postnatal Day 21, and pups were indirectly exposed in utero and throughout lactation. Increased incidence or severity of renal pelvic dilatation was observed in adult offspring of treated dams at 75 mg/kg/day, the highest dose tested (associated maternal and pup dapagliflozin exposures were 1,415 times and 137 times, respectively, the human exposure at the MRHD based on AUC comparisons). Additional developmental toxicity was limited to dose-related reductions in pup body weights, and observed only at doses ≥15 mg/kg/day (associated with pup exposures ≥29 times the human exposure at the MRHD based on AUC comparisons). Maternal toxicity was evident only at the highest dose tested, and limited to transient reductions in body weight and food consumption. The no observed adverse effect level (NOAEL) for developmental toxicity, was 1 mg/kg/day (~19 times the human exposure at the MRHD based on AUC comparisons).
In embryo-fetal development studies in rats and rabbits, dapagliflozin was administered throughout organogenesis, corresponding to the first trimester of human pregnancy. In rats, dapagliflozin was neither embryo lethal nor teratogenic at doses up to 75 mg/kg/day (~1441-times the human exposure at the MRHD, based on AUC comparisons). No maternal or developmental toxicities were observed in rabbits at doses up to 180 mg/kg/day (~1191 times the human exposure at the MRHD, based on AUC comparisons).
Sitagliptin
Renal and liver toxicity were observed in rodents at systemic exposure values 58 times the human exposure level, while the no-effect level was found at 19 times the human exposure level. Incisor teeth abnormalities were observed in rats at exposure levels 67 times the clinical exposure level; the no-effect level for this finding was 58-fold based on the 14-week rat study. The relevance of these findings for humans is unknown. Transient treatment-related physical signs, some of which suggest neural toxicity, such as openmouth breathing, salivation, white foamy emesis, ataxia, trembling, decreased activity, and/or hunched posture were observed in dogs at 50 mg/kg/day (~23 times the human exposure at the MRHD based on AUC comparisons). In addition, very slight to slight skeletal muscle degeneration was also observed histologically in the 3- and 6-month toxicity studies at the 50 mg/kg/day. However, no skeletal muscle degeneration was found in the 12-month toxicity study, indicating the lack of reproducibility or progression of this change with increased duration of treatment. A no-effect level for these findings was 10 mg/kg/day (~6 times the human exposure at the MRHD, based on AUC comparisons).
Sitagliptin has not been demonstrated to be genotoxic in preclinical studies. Sitagliptin was not carcinogenic in mice. In rats, there was an increased incidence of hepatic adenomas and carcinomas (~58 times the human exposure at the MRHD, based on AUC comparisons). Since hepatotoxicity has been shown to correlate with induction of hepatic neoplasia in rats, this increased incidence of hepatic tumors in rats was likely secondary to chronic hepatic toxicity at this high dose. Because of the high safety margin (~19-fold the human exposure at the MRHD, based on AUC comparisons), these neoplastic changes are not considered relevant for the situation in humans. No adverse effects upon fertility were observed in male and female rats given sitagliptin prior to and throughout mating. No adverse effect on fertility was observed at 125 mg/kg/day (~12 times human exposure at the MRHD based on AUC comparisons). In a pre-/postnatal development study performed in rats sitagliptin showed no adverse effects on functional or behavioral toxicity in offspring were noted at doses up to 1000 mg/kg/day
Reproductive toxicity studies showed a slight treatment-related increased incidence of fetal rib malformations (absent, hypoplastic and wavy ribs) in the offspring of rats at systemic exposure levels more than 29 times the human exposure levels. Maternal toxicity was seen in rabbits at more than 29 times the human exposure levels. Because of the high safety margins, these findings do not suggest a relevant risk for human reproduction. Sitagliptin is secreted in considerable amounts into the milk of lactating rats (milk/plasma ratio: 4:1).
Metformin
Preclinical data reveal no special hazard for humans based on conventional studies on safety, pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential and reproductive toxicity.
7.0 Description
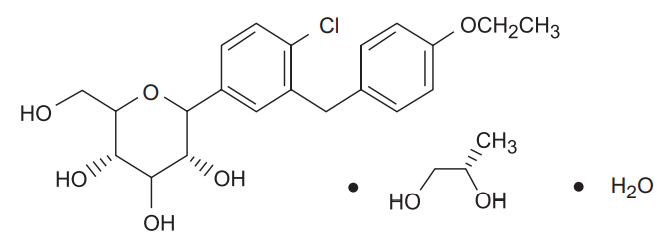
Dapagliflozin
Dapagliflozin Propanediol monohydrate: D-glucitol, 1,5-anhydro-1-C-[4-chloro-3-[(4- ethoxyphenyl)methyl]phenyl]-, (1S)-, compounded with (2S)-1,2-propanediol, hydrate
(1:1:1); It has an empirical formula of C21H25ClO6•C3H8O2•H2O and a molecular weight of 502.98 gm/mole. The Structural formula is :
Sitagliptin
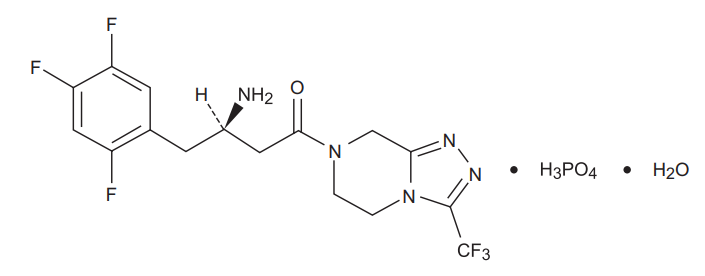
Sitagliptin is an orally-active inhibitor of the dipeptidyl peptidase-4 (DPP-4) enzyme. Sitagliptin is present in the form of sitagliptin phosphate monohydrate. Sitagliptin phosphate monohydrate is described chemically as 7-[(3R)-3-amino-1-oxo-4-(2,4,5- trifluorophenyl)butyl]-5,6,7,8¬tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3- a]pyrazine phosphate (1:1) monohydrate with an empirical formula of C16H15F6N5O•H3PO4•H2O and a molecular weight of 523.32. The structural formula is :
Metformin Hcl
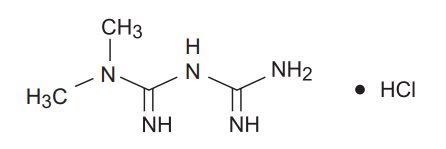
Metformin hydrochloride (N, N-dimethylimidodicarbonimidic diamide hydrochloride) is a white to off-white crystalline compound with a molecular formula of C4H11N5•HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water, slightly soluble in alcohol, and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68.
The structural formula is:
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
Zensita-Trio 500 : Alu-Alu Blister strip of 15 tablets.
Zensita-Trio 1000 : Alu-Alu Blister strip of 15 tablets.
8.4 Storage and handing instructions
Store below 30°C.
Keep out of reach of children.
9.0 Patient counselling information
Do not take Sitagliptin and Metformin Extended Release and Dapagliflozin Tablets : If you are allergic to Dapagliflozin, Sitagliptin and Metformin or any of the other ingredients of this medicine. If you think you may be allergic to Sitagliptin / Dapagliflozin / Metformin or any of the other ingredients of do not take this medicine and talk to your doctor.
Warnings and precautions :
Talk to your doctor, before taking Sitagliptin / Dapagliflozin / Metformin :
- if you have type 1 diabetes (i.e., your body does not produce insulin) or if you have a condition called diabetic ketoacidosis.
- if you are taking an anti-diabetic medicine known as sulphonylurea (your doctor may want to reduce your dose of the sulphonylurea when you take it together with Sitagliptin in order to avoid low blood glucose [hypoglycaemia]).
- if you have moderate or severe kidney disease (you will need to take a lower dose of this combination).
- if you are on dialysis.
- if you have liver disease.
- if you suffer from heart failure.
- if you have or have had a disease of the pancreas.
- if you have malaise, myalgia, abdominal pain, respiratory distress, or increased somnolence; however, hypotension and resistant Brady arrhythmias have occurred with severe acidosis.
- if you have significant renal impairment
- If you have previously taken Sitagliptin / Dapaglilozin/ Metformin individual components but had stopped taking it because of liver disease, you should not take this combination.
Major side effects to be explained
Lactic acidosis, pancreatitis etc.
Diabetic skin lesions are a common complication of diabetes. You are advised to follow the recommendations for skin and foot care that you are given by your doctor or nurse. You are also advised to pay particular attention to new onset of blisters or ulcers while taking Sitagliptin. Should these occur, you should promptly consult your doctor. A test to determine your liver function will be performed before the start of Sitagliptin treatment, at three-month intervals for the first year and periodically thereafter. This is so that signs of increased liver enzymes can be detected as early as possible.
Children and adolescents
The use of this combination product in children and adolescents up to 18 years of age is not recommended.
Other medicines and Sitagliptin and Metformin Extended Release and Dapagliflozin Tablets
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Your doctor may wish to alter your dose of Sitagliptin if you are taking other medicines such as :
- Thiazides or other diuretics (also called water tablets)
- Corticosteroid (generally used to treat inflammation)
- Thyroid medicines
- Certain medicines affecting the nervous system.
Pregnancy, breast feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
If you feel dizzy while taking this medicine, do not drive or use machines. How should I take Dapagliflozin and Sitagliptin and Metformin Extended-Release Tablets?
Swallow the tablets whole with some water once daily.
If you have taken too many tablets?
Contact your doctor immediately or go to the nearest hospital casualty department taking any remaining medication and this patient information leaflet with you.
If you forget to take Dapagliflozin and Sitagliptin and Metformin Extended-Release Tablets?
If you miss a dose of Dapagliflozin, Sitagliptin and Metformin Extended-Release Tablets, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular schedule. Do not double the dose.
What are the possible side effects of Dapagliflozin, Sitagliptin and Metformin Extended-Release Tablets?
Like all medicines, this medicine can cause side effects, although not everybody gets them. Stop taking this medicine and consult your doctor immediately if any of the following occur-you may need medical treatment. The most common side effects with the combination include nasopharyngitis and headache.
12.0 Date of issue
18 November 2022
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What Zensita Trio is and what it is used for
- What you need to know before you take Zensita Trio
- How to take Zensita Trio
- Possible side effects
- How to store Zensita Trio
- Contents of the pack and other information
1. What Zensita Trio is and what it is used for
It is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Zensita Trio contains three different medicines called dapagliflozin, sitagliptin and metformin.
- Dapagliflozin belongs to a class of medicines known as sodium-glucose cotransporter-2 (SGLT2) inhibitors.
- Sitagliptin belongs to a class of medicines called DPP-4 inhibitors (dipeptidyl peptidase-4 inhibitors)
- Metformin belongs to a class of medicines called biguanides.
They work together to control blood sugar levels in adult patients with a form of diabetes called ‘type 2 diabetes mellitus’. This medicine helps to increase the levels of insulin produced after a meal and lowers the amount of sugar made by your body, as well as reduce the blood sugar level.
Along with diet and exercise, this medicine helps lower your blood sugar.
What is type 2 diabetes?
Type 2 diabetes is a condition in which your body does not make enough insulin, and the insulin that your body produces does not work as well as it should. Your body can also make too much sugar. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems like heart disease, kidney disease, blindness, and amputation.
2. What you need to know before you take Zensita Trio
Do not take Zensita Trio
- if you are allergic to dapagliflozin, sitagliptin or metformin or any of the other ingredients of this medicine
- if you have severely reduced kidney function
- if you have uncontrolled diabetes, with e.g. severe hyperglycaemia (high blood glucose), nausea, vomiting, diarrhoea, rapid weight loss, lactic acidosis (see “Risk of lactic acidosis” below) or ketoacidosis. Ketoacidosis is a condition in which substances called ‘ketone bodies’ accumulate in the blood and which can lead to diabetic pre-coma. Symptoms include stomach pain, fast and deep breathing, sleepiness or your breath developing an unusual fruity smell.
- if you have a severe infection or are dehydrated
- if you are going to have an X-ray where you will be injected with a dye. You will need to stop taking Zensita Trio at the time of the X-ray and for 2 or more days after as directed by your doctor, depending on how your kidneys are working.
- if you have recently had a heart attack or have severe circulatory problems, such as ‘shock’ or breathing difficulties
- if you have liver problems
- if you drink alcohol to excess (either every day or only from time to time)
- if you are breast-feeding
Do not take Zensita Trio if any of the above apply to you and talk with your doctor about other ways of managing your diabetes. If you are not sure, talk to your doctor, pharmacist or nurse before taking Zensita Trio.
Warnings and precautions
Cases of inflammation of the pancreas (pancreatitis) have been reported in patients receiving Zensita Trio. If you encounter blistering of the skin it may be a sign for a condition called bullous pemphigoid. Your doctor may ask you to stop Zensita Trio.
Risk of lactic acidosis
Zensita Trio may cause a very rare, but very serious side effect called lactic acidosis, particularly if your kidneys are not working properly. The risk of developing lactic acidosis is also increased with uncontrolled diabetes, serious infections, prolonged fasting or alcohol intake, dehydration, liver problems and any medical conditions in which a part of the body has a reduced supply of oxygen (such as acute severe heart disease).
If any of the above apply to you, talk to your doctor for further instructions.
Stop taking Zensita Trio for a short time if you have a condition that may be associated with dehydration (significant loss of body fluids) such as severe vomiting, diarrhoea, fever, exposure to heat or if you drink less fluid than normal. Talk to your doctor for further instructions. Stop taking Zensita Trio and contact a doctor or the nearest hospital immediately if you experience some of the symptoms of lactic acidosis, as this condition may lead to coma.
Symptoms of lactic acidosis include:
- vomiting
- stomach ache (abdominal pain)
- muscle cramps
- a general feeling of not being well with severe tiredness
- difficulty in breathing
- reduced body temperature and heartbeat
Lactic acidosis is a medical emergency and must be treated in a hospital.
Talk to your doctor or pharmacist before taking Zensita Trio:
- if you have or have had a disease of the pancreas (such as pancreatitis)
- if you have or have had gallstones, alcohol dependence or very high levels of triglycerides (a form of fat) in your blood. These medical conditions can increase your chance of getting pancreatitis. - if you have type 1 diabetes. This is sometimes called insulin-dependent diabetes
- if you have or have had an allergic reaction to sitagliptin, metformin, or Zensita Trio.
- if you are taking a sulphonylurea or insulin, diabetes medicines, together with Zensita Trio, as you may experience low blood sugar levels (hypoglycaemia). Your doctor may reduce the dose of your sulphonylurea or insulin.
If you need to have major surgery you must stop taking Zensita Trio during and for some time after the procedure. Your doctor will decide when you must stop and when to restart your treatment with Zensita Trio.
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before taking Zensita Trio.
During treatment with Zensita Trio, your doctor will check your kidney function at least once a year or more frequently if you are elderly and/or if you have worsening kidney function.
Children and adolescents
Children and adolescents below 18 years should not use this medicine. It is not effective in children and adolescents between the ages of 10 and 17 years. It is not known if this medicine is safe and effective when used in children younger than 10 years.
Other medicines and Zensita Trio
If you need to have an injection of a contrast medium that contains iodine into your bloodstream, for example, in the context of an X-ray or scan, you must stop taking Zensita Trio before or at the time of the injection. Your doctor will decide when you must stop and when to restart your treatment with Zensita Trio.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. You may need more frequent blood glucose and kidney function tests, or your doctor may need to adjust the dosage of Zensita Trio. It is especially important to mention the following:
- medicines (taken by mouth, inhalation, or injection) used to treat diseases that involve inflammation, like asthma and arthritis (corticosteroids)
- medicines which increase urine production (diuretics)
- medicines used to treat pain and inflammation (NSAID and COX-2-inhibitors, such as ibuprofen and celecoxib)
- certain medicines for the treatment of high blood pressure (ACE inhibitors and angiotensin II receptor antagonists)
- specific medicines for the treatment of bronchial asthma (β-sympathomimetic)
- iodinated contrast agents or alcohol-containing medicines
- certain medicines used to treat stomach problems such as cimetidine
- ranolazine, a medicine used to treat angina
- dolutegravir, a medicine used to treat HIV infection
- vandetanib, a medicine used to treat a specific type of thyroid cancer (medullary thyroid cancer)
- digoxin (to treat irregular heartbeat and other heart problems). The level of digoxin in your blood may need to be checked if taking with Zensita Trio.
Zensita Trio with alcohol
Avoid excessive alcohol intake while taking Zensita Trio since this may increase the risk of lactic acidosis (see section “Warnings and precautions”).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. You should not take this medicine during pregnancy or if you are breast-feeding. See section 2, Do not take Zensita Trio.
Driving and using machines
This medicine has no or negligible influence on the ability to drive and use machines. However, dizziness and drowsiness have been reported with sitagliptin, which may affect your ability to drive or use machines. Taking this medicine in combination with medicines called sulphonylureas or with insulin can cause hypoglycaemia, which may affect your ability to drive and use machines or work without safe foothold.
3. How to take Zensita Trio
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
- Take one tablet:
- once daily by mouth
- with meals to lower your chance of an upset stomach.
- Your doctor may need to increase your dose to control your blood sugar.
- If you have reduced kidney function, your doctor may prescribe a lower dose.
You should continue the diet recommended by your doctor during treatment with this medicine and take care that your carbohydrate intake is equally distributed over the day.
This medicine alone is unlikely to cause abnormally low blood sugar (hypoglycaemia). When this medicine is used with a sulphonylurea medicine or with insulin, low blood sugar can occur and your doctor may reduce the dose of your sulphonylurea or insulin.
If you take more Zensita Trio than you should
If you take more than the prescribed dosage of this medicine, contact your doctor immediately. Go to the hospital if you have symptoms of lactic acidosis such as feeling cold or uncomfortable, severe nausea or vomiting, stomach ache, unexplained weight loss, muscular cramps, or rapid breathing.
If you forget to take Zensita Trio
If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take a double dose of this medicine.
If you stop taking Zensita Trio
Continue to take this medicine as long as your doctor prescribes it so you can continue to help control your blood sugar. You should not stop taking this medicine without talking to your doctor first. If you stop taking Zensita Trio, your blood sugar may rise again. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
STOP taking Zensita Trio and contact a doctor immediately if you notice any of the following serious side effects:
- Severe and persistent pain in the abdomen (stomach area) which might reach through to your back with or without nausea and vomiting, as these could be signs of an inflamed pancreas (pancreatitis).
- Zensita Trio may cause a very rare (may affect up to 1 in 10,000 people), but very serious side effect called lactic acidosis (see section “Warnings and precautions”). If this happens, you must stop taking Zensita Trio and contact a doctor or the nearest hospital immediately, as lactic acidosis may lead to coma.
- If you have a serious allergic reaction (frequency not known), including rash, hives, blisters on the skin/peeling skin and swelling of the face, lips, tongue, and throat that may cause difficulty in breathing or swallowing, stop taking this medicine and call your doctor right away. Your doctor may prescribe a medicine to treat your allergic reaction and a different medicine for your diabetes.
Some patients taking metformin have experienced the following side effects after starting sitagliptin:
Common (may affect up to 1 in 10 people): low blood sugar, nausea, flatulence, vomiting.
Uncommon (may affect up to 1 in 100 people): stomach ache, diarrhoea, constipation, drowsiness.
Some patients have experienced diarrhoea, nausea, flatulence, constipation, stomach ache or vomiting when starting the combination of sitagliptin and metformin together (frequency is common).
Very common (may affect more than 1 in 10 people): low blood sugar
Common: constipation
Some patients have experienced the following side effects during clinical studies while taking sitagliptin alone (one of the medicines in Zensita Trio) or during post-approval use of Zensita Trio or sitagliptin alone or with other diabetes medicines:
Common: low blood sugar, headache, upper respiratory infection, stuffy or runny nose and sore throat, osteoarthritis, arm or leg pain
Uncommon: dizziness, constipation, itching
Rare: reduced number of platelets
Frequency not known: kidney problems (sometimes requiring dialysis), vomiting, joint pain, muscle pain, back pain, interstitial lung disease, bullous pemphigoid (a type of skin blister)
Some patients have experienced the following side effects while taking metformin alone:
Very common: nausea, vomiting, diarrhoea, stomach ache and loss of appetite. These symptoms may happen when you start taking metformin and usually go away
Common: a metallic taste
Very rare: decreased vitamin B12 levels, hepatitis (a problem with your liver), hives, redness of the skin (rash) or itching
Infections & infestations:
Common: genital infections, urinary tract infection
Uncommon: Fungal infection
Reporting of side effects If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Zensita Trio
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the blister and the carton after 'EXP'.
- The expiry date refers to the last day of the month.
- Do not store above 30°C.
- Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Zensita Trio contains
- The active substances are dapagliflozin, sitagliptin and metformin.
- Each Zensita Trio 10mg/ 100mg/ 500 mg film-coated tablet contains dapagliflozin 10mg, sitagliptin phosphate monohydrate equivalent to 100 mg of sitagliptin and 500 mg of metformin hydrochloride
- Each Zensita Trio 10mg/ 100mg/ 1,000 mg mg film-coated tablet contains dapagliflozin 10mg, sitagliptin phosphate monohydrate equivalent to 100 mg of sitagliptin and 1,000 mg of metformin hydrochloride.
Packaging information
Zensita-Trio 500: Alu-Alu Blister strip of 15 tablets each.
Zensita-Trio 1000: Alu-Alu Blister strip of 15 tablets each.
Marketing Authorisation Holder
Zuventus Healthcare Limited Zuventus House Plot No. Y2, CTS No: 358/A2, near Nahur Railway Station, off Raycon IT Park Road, Nahur West, Mumbai, Maharashtra 400078
This leaflet was last revised in July 2023.