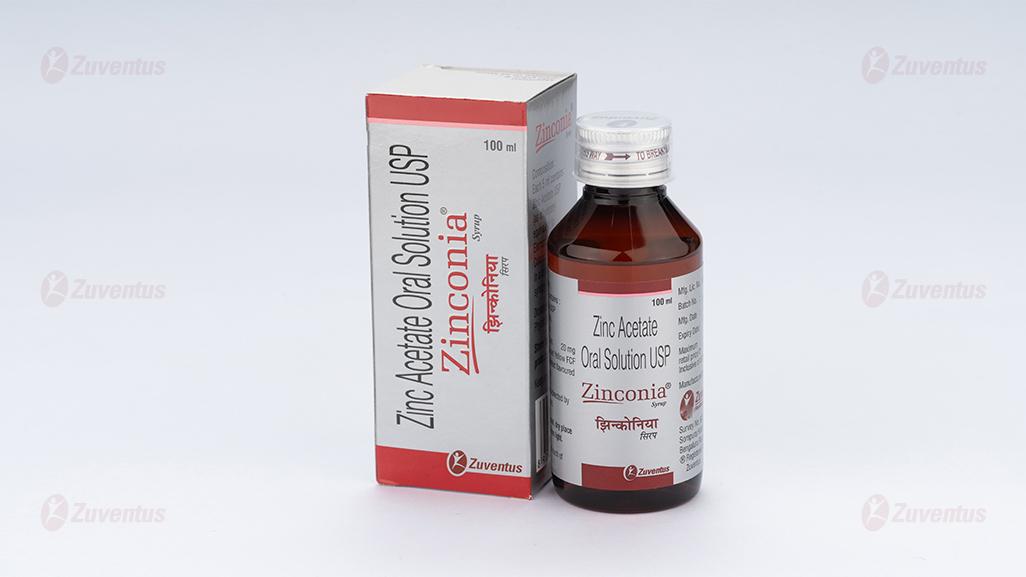
Zinconia Syrup
Therapy Area
Vitamins/Minerals Supplements
1.0 Generic name
Zinc Acetate Oral Solution USP
2.0 Qualitative and quantitative composition
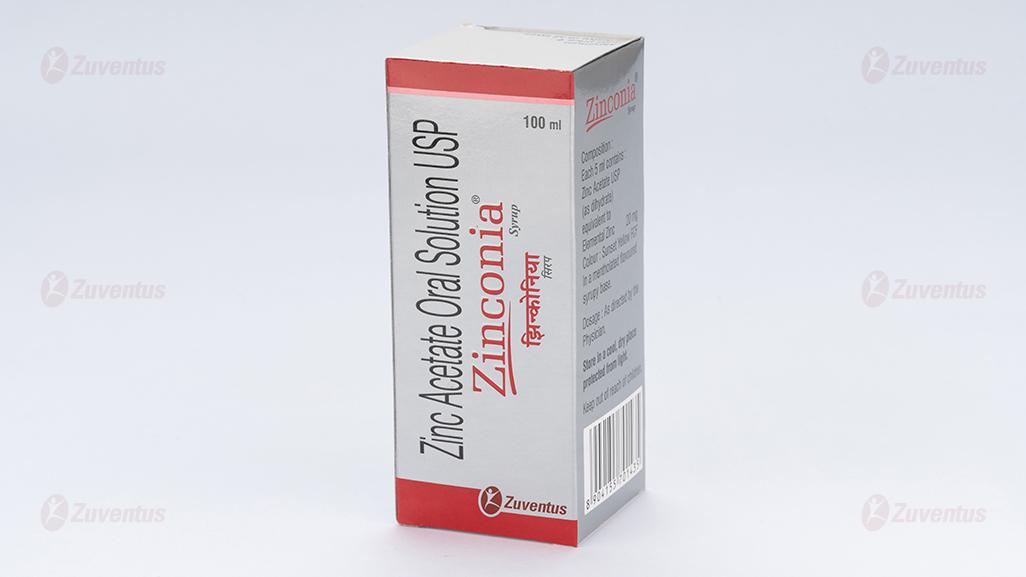
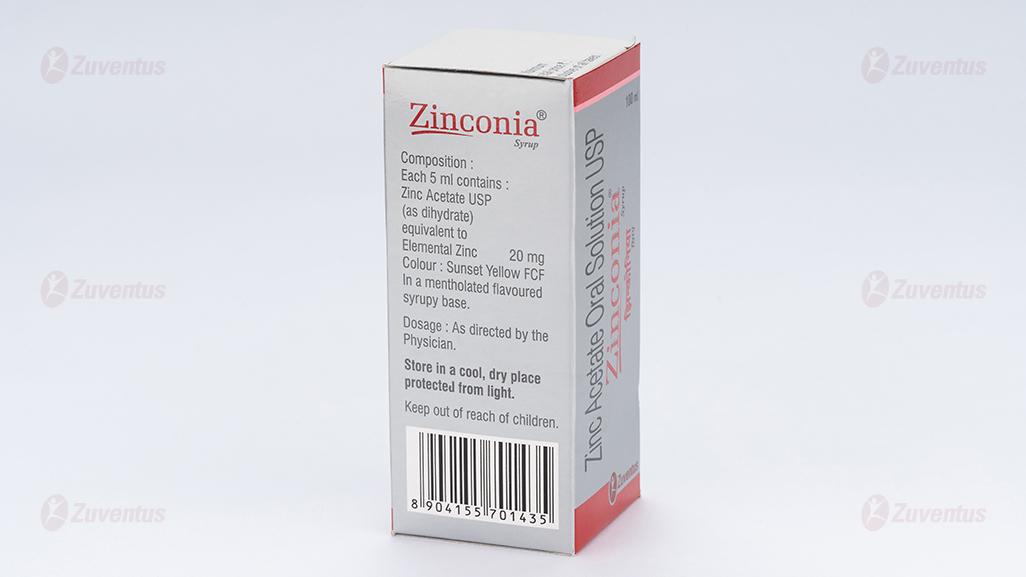
Each 5ml contains:
Zinc acetate USP (as dihydrate) equivalent to Elemental Zinc 20 mg
Colour: Sunset Yellow FCF.
In a mentholated flavoured syrup base.
3.0 Dosage form and strength
Dosage form - Syrup
Dosage Strength –Elemental Zinc 20 mg/5 ml
4.0 Clinical particulars
4.1 Therapeutic indication
For the treatment of acute diarrhoea in children as an adjunct to oral rehydration.
4.2 Posology and method of administration
As per WHO/UNICEF recommendations zinc supplementation can be used orally, as an adjunct to Oral Rehydration Therapy in acute diarrhea in following dose regimen:
In infants (under six months): 2.5ml (10 mg of elemental Zinc) daily for 10–14 days after meals
In children (children older than six months): 5 ml (20 mg of elemental Zinc) daily for 10 –14 days after meals
4.3 Contraindications
- Hypersensitivity to zinc salts or any component of a zinc-containing supplement.
- Copper deficiency
4.4 Special warnings and precautions for use
Renal impairment: Accumulation of zinc may occur in cases of renal failure. Hence caution should be exercised in patients with renal impairment with careful patient monitoring
4.5 Drugs interactions
Copper: Zinc may inhibit the absorption of copper.
Tetracyclines: Zinc may reduce the absorption of concurrently administered tetracyclines, also the absorption of zinc may be reduced by tetracyclines; when both are being given an interval of at least three hours should be allowed.
Quinolone Antibacterials: Zinc may reduce the absorption of quinolones; ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin and ofloxacin.
Calcium Salts: The absorption of zinc may be reduced by calcium salts.
Iron: The absorption of zinc may be reduced by oral iron, also the absorption of oral iron may be reduced by zinc.
Penicillamine: The absorption of zinc may be reduced by penicillamine, also the absorption of penicillamine may be reduced by zinc. Trientine: The absorption of zinc may be reduced by trientine, also the absorption of trientine may be reduced by zinc.
4.6 Use in special populations
Pregnancy
The safety of this product in human pregnancy has not been established. Zinc crosses the placenta and is present in breast milk.
Lactation
Zinc is excreted in human breast milk and zinc-induced copper deficiency in the breast-fed baby may occur. Therefore, breast-feeding should be avoided during zinc therapy.
4.7 Effects on ability to drive and use machines
Zinconia® Syrup has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Zinc salts may cause abdominal pain, dyspepsia, nausea, vomiting, diarrhoea, gastric irritation and gastritis. There have also been cases of irritability, headache and lethargy observed. Zinc may interfere with the absorption of copper, leading to reduced copper levels, and potentially copper deficiency. The risk of copper deficiency may be greater with long-term treatment (e.g. if zinc deficiency is no longer present) and/or with higher doses of zinc.
Reporting of suspected adverse reactions
- Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com '
- Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.8 Overdose
Zinc acetate is corrosive in overdosage. Symptoms are corrosion and inflammation of the mucous membrane of the mouth and stomach; ulceration of the stomach followed by perforation may occur. Gastric lavage and emesis should be avoided. Demulcents such as milk should be given. Chelating agents such as sodium calcium edetate may be useful.
5.0 Pharmacological properties
5.1 Mechanism of Action
Zinc supplementation improves immunity. Various studies have quoted different mechanisms of action of zinc in persistent and infective diarrhea patients: Preclinical studies have shown that zinc inhibits cAMP induced, chloride dependent fluid secretion by inhibiting basolateral potassium (K) channels of ileal mucosa. Zinc also improves the absorption of water and electrolytes, improves regeneration of the intestinal epithelium, increases the levels of brush border enzymes, and enhances the immune response, allowing for a better clearance of the pathogens. Recent evidence demonstrate that zinc inhibits toxin induced cholera. Thus by different mechanisms zinc supplementation could be beneficial in diarrhea.
It is demonstrated that zinc supplementation could improve symptoms of acute respiratory illness. It may prevent premature cell destruction and promotes activity of enzymes that affect production of prostaglandin from essential fatty acids, and as a result, leads to decrement of inflammation in airways. It also can activate natural killer cells, macrophages and lymphocytes. Zinc administration has also been demonstrated to improve tachypnea during acute phase of Acute Lower Respiratory Tract Infections in hospitalized children.
Zinc has critical effect in homeostasis, in immune function, oxidative stress, apoptosis, and aging, and significant disorders of great public health interest are associated with zinc deficiency. In many chronic diseases, including atherosclerosis, several malignancies, neurological disorders, autoimmune diseases, aging, age-related degenerative diseases, and Wilson's disease, the concurrent zinc deficiency may complicate the clinical features, affect adversely immunological status, increase oxidative stress, and lead to the generation of inflammatory cytokines. In these diseases, oxidative stress and chronic inflammation may play important causative roles.
5.2 Pharmacodynamic properties
Zinc is an essential trace element involved in many enzyme systems. Severe deficiency causes skin lesion, alopecia, diarrhoea, increased susceptibility to infections and failure to thrive in children. Symptoms of less severe deficiency include distorted or absent perceptions of taste and smell and poor wound healing.
5.3 Pharmacokinetic properties
Zinc is absorbed (20% to 30%) from the gastrointestinal tract and distributed throughout the body. The highest concentrations occur in hair, eyes, male reproductive organs and bone. Lower levels are present in liver, kidney and muscle. In blood 80% is found in erythrocytes. Plasma zinc levels range from 70 to 110μg/dL and about 50% of this is loosely bound to albumin. About 7% is amino-acid bound and the rest is tightly bound to alpha 2-macroglobulins and other proteins. The liver is the main storage for zinc and hepatic zinc levels are increased during maintenance therapy with zinc.
The plasma elimination half-life of zinc in healthy subjects is around 1 hour. The elimination of zinc results primarily from faecal excretion with relatively little from urine and sweat. The faecal excretion is in the greatest part due to the passage of unabsorbed zinc but it is also due to endogenous intestinal secretion.
6.0 Nonclinical properties
None stated.
7.0 Description
Zinc acetate as the dihydrate is a salt of zinc used for the treatment of acute diarrhoea in children as an adjunct to oral rehydration.
Zinc is an integral component of many metallo enzymes in the body; it is involved in the synthesis and stabilization of proteins, DNA and RNA, and plays a structural role in ribosomes and membranes. Zinc is involved in oxygen transport and protection against free radical damage. Zinc facilitates wound healing and helps maintain normal growth rates, normal skin hydration and senses of taste and smell. Zinc acts as an integral part of several enzymes important to protein and carbohydrate metabolism.
Its structural formula is
Zinc acetate dihydrate structure
Molecular Formula: C4H6O4Zn.2H2O
Molecular Weight: 219.5 g/mol
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None.
8.2 Shelf-life
Refer to pack.
8.3 Packaging information
Amber-coloured bottle of 100 mL.
8.4 Storage and handing instructions
Store in a cool, dry place protected from light.
Keep out of reach of children.
9.0 Patient Counselling Information
Do not take Zinconia® Syrup
- if you are allergic (hypersensitive) to zinc acetate or any of the other ingredients.
- if you have copper deficiency
Talk to your doctor, before taking Zinconia® Syrup if you suffer from kidney disease. If this applies to you it is important that you tell your doctor or pharmacist before taking Zinconia® Syrup and they will decide what to do.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This is especially important if you are taking or have recently taken any of the following:
- Copper supplements
- Tetracycline antibiotics (such as oxytetracycline or doxycycline) used to treat certain bacterial infections
- Quinolone antibiotics (such as ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin and ofloxacin) used to treat certain bacterial infections
- Calcium Salt Preparations
- Penicillamine (used to treat rheumatoid arthritis, Wilson's disease, autoimmune hepatitis and cystinuria)
- Trientine (used in the treatment of Wilson's disease)
Zinconia® Syrup should be taken after meals. Patients should take Zinconia® Syrup two to three hours after meals. In the rare event of gastric intolerance of zinc, generally occurring with the morning dose, this dose may be taken between breakfast and lunch.
12.0 Date of revision
16th Oct. 2023
About leaflet
Read all of this leaflet carefully before your child starts using this medicine, because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, please ask your child’s doctor, pharmacist or nurse.
- This medicine has been prescribed for your child only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as your child’s.
- If your child gets any side effects, talk to your child’s doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4
What is in this leaflet
- What ZINCONIA® Syrup is and what it is used for
- What you need to know before you use ZINCONIA® Syrup
- How to use ZINCONIA® Syrup
- Possible side effects
- How to store ZINCONIA® Syrup
- Contents of the pack and other information
1. What ZINCONIA® Syrup is and what it is used for
ZINCONIA® Syrup is a mineral supplement used for the treatment of acute diarrhoea in children as an adjunct to oral rehydration. It contains zinc acetate (as dihydrate). Zinc acetate dihydrate is a source of zinc, which is an essential trace element and involved in a number of body enzyme functions.
Zinc is an essential trace element involved in many enzyme systems. Severe deficiency causes skin lesion, alopecia, diarrhoea, increased susceptibility to infections and failure to thrive in children. Symptoms of less severe deficiency include distorted or absent perceptions of taste and smell and poor wound healing.
You must talk to a doctor if you do not feel better or if you feel worse.
2. Before you take ZINCONIA® Syrup
Do not take ZINCONIA® Syrup if you
- if you are allergic (hypersensitive) to zinc or to any of the components of the formulation.
- if you have copper deficiency
Talk to your doctor if this applies to you.
Warnings and precautions
- Talk to your doctor, pharmacist or nurse before taking ZINCONIA® syrup if you suffer from kidney disease.
- If this applies to you it is important that you tell your doctor or pharmacist before taking ZINCONIA® syrup and they will decide what to do. It may still be safe for you to take syrup.
Taking other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
This is especially important if you are taking or have recently taken any of the following:
-copper supplements (see section 2 “Do not take ZINCONIA® ….’)
-tetracycline antibiotics (such as oxytetracycline or doxycycline) used to treat certain bacterial infections
-quinolone antibiotics (such as ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin and ofloxacin) used to treat certain bacterial infections
-calcium salt preparations
-iron preparations
-penicillamine (used to treat rheumatoid arthritis, Wilson’s disease, autoimmune hepatitis and cystinuria)
-trientine (used in the treatment of Wilson’s disease)
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Taking ZINCONIA® Syrup with food and drink
You should take ZINCONIA® Syrup after meals.
Pregnancy and breast-feeding
The safety of ZINCONIA® Syrup in human pregnancy is not known. Zinc has been shown to cross the placenta and is present in breast milk in females taking zinc supplements. Only take this product during pregnancy or while breast-feeding if your doctor has advised you to do so.
Ask your doctor or pharmacist for advice before taking any medicine during pregnancy or while breast-feeding.
Driving and using machines
ZINCONIA® Syrup is not expected to affect the ability to drive or use machine.
3. How to take ZINCONIA® Syrup
Always take ZINCONIA® Syrup exactly as your doctor has told you. It should be taken orally.
Dose
Acute Diarrhoea:
- For children below 6 months: 2.5 ml daily for 10-14 days
- For children above 6 months: 5 ml daily for 10-14 days
If you take more ZINCONIA® Syrup than you should
If you take too large dosage, contact your nearest hospital casualty department or doctor immediately.
If you forget to take ZINCONIA® Syrup
If you forget to take your dose, take it as soon as you remember and then continue with the next dose as instructed. Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
If you stop taking ZINCONIA® Syrup
To get the most benefit from ZINCONIA® Syrup, always finish the course of treatment recommended by your doctor or pharmacist.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, ZINCONIA® Syrup can cause side effects, although not everybody gets them.
Side effects with ZINCONIA® Syrup may include:
- reduced copper levels, potentially leading to copper deficiency
- abdominal pain -indigestion
- nausea (feeling sick)
- vomiting (being sick)
- diarrhoea
- stomach discomfort
- irritability
- headache
- lethargy (a feeling of weariness)
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store ZINCONIA® Syrup
Store in a cool, dry place protected from light.
Keep out of reach of children.
Do not use this medicine after the expiry date which is stated on the carton and tablet container.
The expiry date refers to the last day of that month.
Store in the original packaging to protect the tablets from moisture.
Do not take ZINCONIA® Syrup if you notice that the bottle is damaged.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What ZINCONIA® Syrup contains
Each 5 ml contains:
Zinc acetate USP (as dihydrate) equivalent to Elemental Zinc 20 mg
Colour: Sunset Yellow FCF.
In a mentholated flavoured syrup base
What ZINCONIA® Syrup looks like and contents of pack
Amber-coloured bottle of 100 mL with measuring cap