Zosa Junior Granules
Therapy Area
Gastrointestinal
1.0 Generic name
Esomeprazole Delayed Release Granules for Oral Suspension
2.0 Qualitative and quantitative composition
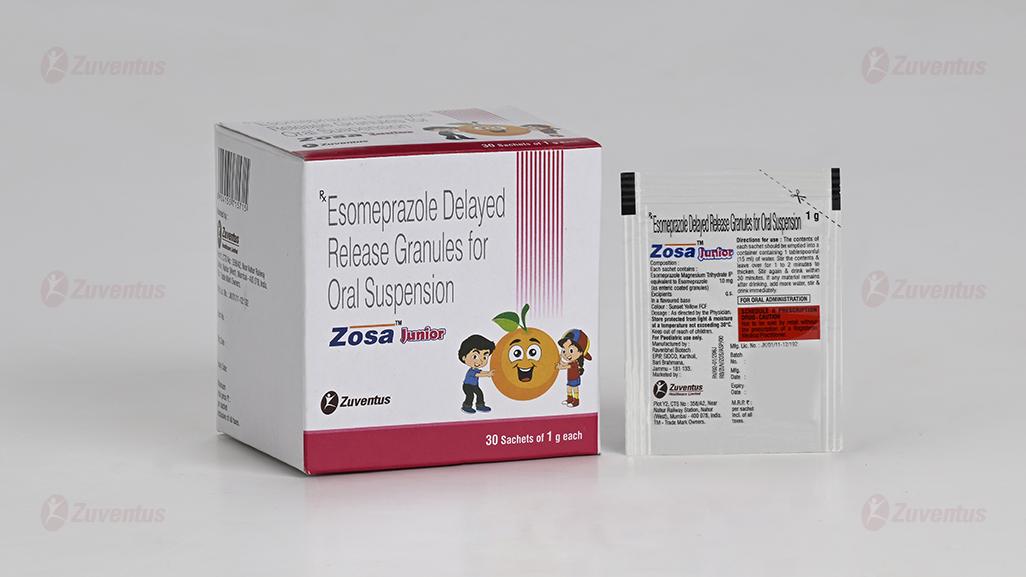
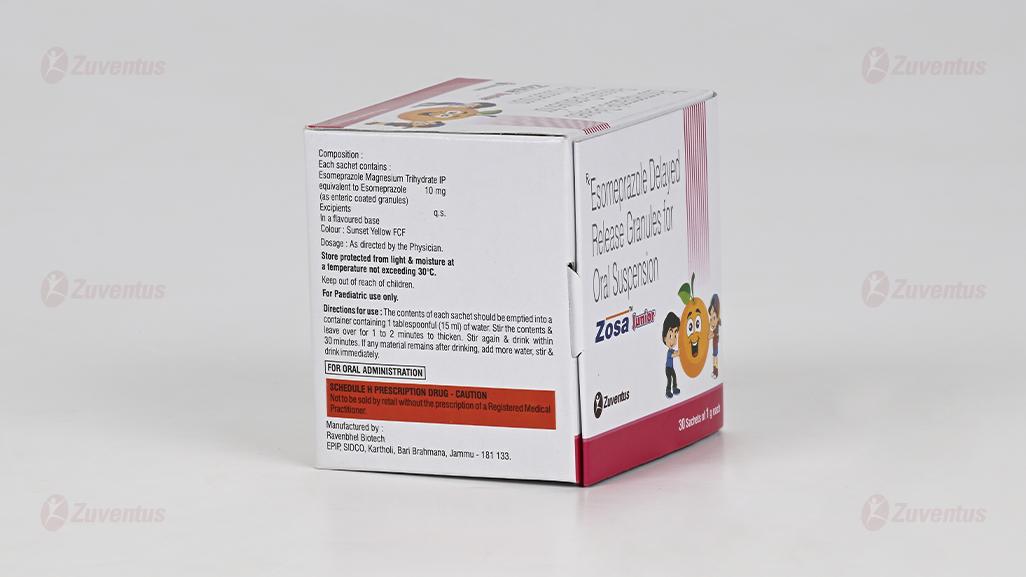
Each sachet contains :
Esomeprazole Magnesium Trihydrate IP
equivalent to Esomeprazole 10 mg
(as enteric coated granules)
Colour : Sunset Yellow FCF
3.0 Dosage form and strength
Delayed release granules for oral suspension 10 mg
4.0 Clinical particulars
4.1Therapeutic Indication
- Treatment of Gastroesophageal Reflux Disease (GERD).
- Risk Reduction of NSAID Associated Gastric Ulcer.
- H. Pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
- Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
4.2 Posology & Method of Administration
Children 1 – 11 years with a bodyweight of ≥10 kg
Gastroesophageal Reflux Disease (GERD)
Treatment of endoscopically proven erosive reflux esophagitis
- Weight ≥10 - <20 kg: 10 mg once daily for 8 weeks.
- Weight ≥20 kg: 10 mg or 20 mg once daily for 8 weeks
Symptomatic treatment of gastroesophageal reflux disease (GERD)
- 10 mg once daily for up to 8 weeks.
Doses over 1 mg/kg/day have not been studied
Children over 4 years of age
Treatment of duodenal ulcer caused by Helicobacter pylori
When selecting appropriate combination therapy, consideration should be given to official national, regional and local guidance regarding bacterial resistance, duration of treatment (most commonly 7 days but sometimes up to 14 days), and appropriate use of antibacterial agents. The treatment should be supervised by a specialist.
The posology recommendation is:
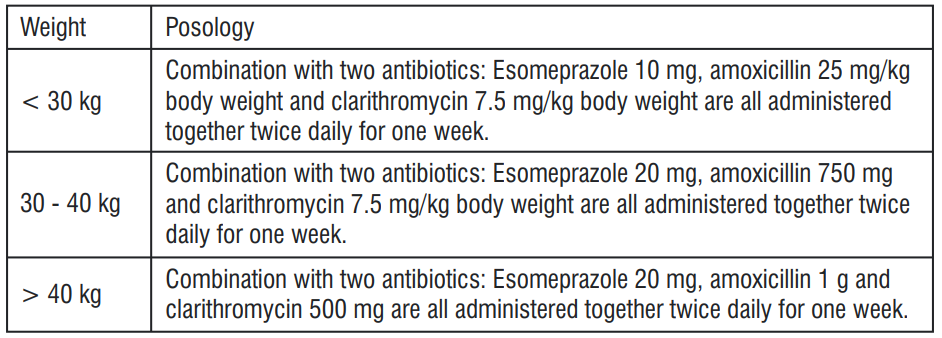
Children below the age of 1 year
The experience of treatment with esomeprazole in infants < 1 year is limited and treatment is therefore not recommended.
Special populations
Renal impairment
Dose adjustment is not required in patients with impaired renal function. Due to limited experience in patients with severe renal insufficiency, such patients should be treated with caution.
Hepatic impairment
Dose adjustment is not required in patients with mild to moderate liver impairment. For patients ≥12 years with severe liver impairment, a maximum dose of 20 mg Esomeprazole should not be exceeded. For children 1-11 years with severe liver impairment, a maximum dose of 10 mg should not be exceeded.
Elderly
Dose adjustment is not required in the elderly
Method of administration
For a 10 mg dose empty the contents of a 10 mg sachet into a glass containing 15 ml water. For a 20 mg dose empty the contents of two 10 mg sachets into a glass containing 30 ml water. Do not use carbonated water. Stir the contents until the granulate has been dispersed and leave for a few minutes to thicken. Stir again and drink within 30 minutes. The granules must not be chewed or crushed. Rinse with 15 ml water to obtain all granules.
- For a 10 mg dose, add the contents of a 10 mg sachet into 15 ml of water.
- For a 20 mg dose add the contents of two 10 mg sachets into 30 ml of water.
- Stir.
- Leave for a few minutes to thicken.
- Stir again.
- Draw the suspension into a syringe.
- Inject through the enteric tube, French size 6 or larger, into the stomach within 30 minutes after reconstitution.
- Refill the syringe with 15 ml water for a 10 mg dose and 30 ml for a 20 mg dose.
- Shake and flush any remaining contents from the enteric tube into the stomach.
- Any unused suspension should be discarded.
4.3 Contraindications
- Hypersensitivity to the active substance, to substituted benzimidazoles or to any of the excipients listed in the formulation.
- should not be used concomitantly with nelfinavir
4.4 Warning & Precautions
In the presence of any alarm symptom (e.g. significant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with Zosa Junior may alleviate symptoms and delay diagnosis.
Long term use
Patients on long-term treatment (particularly those treated for more than a year) should be kept under regular surveillance. Long-term treatment is indicated in adults and adolescents (12 years and older).
On demand treatment
Patients on on-demand treatment should be instructed to contact their physician if their symptoms change in character. On demand treatment has not been investigated in children and is therefore not recommended in this patient group.
Helicobacter Pylori eradication
When prescribing esomeprazole for eradication of Helicobacter pylori possible drug interactions for all components in the triple therapy should be considered. Clarithromycin is a potent inhibitor of CYP3A4 and hence contraindications and interactions for clartithromycin should be considered when triple therapy is used in patients concurrently taking other drugs metabolised via CYP3A4, such as cisapride.
Gastrointestinal infections
Treatment with proton pump inhibitors may lead to a slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter.
Absorption of vitamin B12
Esomeprazole, as all acid-blocking medicines, may reduce the absorption of vitamin B12 (cyanocobalamin) due to hypo or achlorhydria. This should be considered in patients with reduced body stores or risk factors for reduced vitamin B12 absorption on long-term therapy.
Hypomagnesaemia
Severe hypomagnesaemia has been reported in patients treated with proton pump inhibitors (PPIs) like esomeprazole for at least three months, and in most cases for a year. Serious manifestations of hypomagnesaemia such as fatigue, tetany, delirium, convulsions, dizziness and ventricular arrhythmia can occur but they may begin insidiously and be overlooked. In most affected patients, hypomagnesaemia improved after magnesium replacement and discontinuation of the PPI. For patients expected to be on prolonged treatment or who take PPIs with digoxin or medicinal products that may cause hypomagnesaemia (e.g. diuretics), healthcare professionals should consider measuring magnesium levels before starting PPI treatment and periodically during treatment.
Risk of fractures
Proton pump inhibitors, especially if used in high doses and over long durations (>1 year), may modestly increase the risk of hip, wrist and spine fracture, predominantly in the elderly or in presence of other recognised risk factors. Observational studies suggest that proton pump inhibitors may increase the overall risk of fracture by 10-40%. Some of this increase may be due to other risk factors. Patients at risk of osteoporosis should receive care according to current clinical guidelines and they should have an adequate intake of vitamin D and calcium.
Subacute cutaneous lupus erythematosus (SCLE)
Proton pump inhibitors are associated with very infrequent cases of SCLE. If lesions occur, especially in sun-exposed areas of the skin, and if accompanied by ar thralgia, the patient should seek medical help promptly and the health care professional should consider stopping Zosa Junior. SCLE after previous treatment with a proton pump inhibitor may increase the risk of SCLE with other proton pump inhibitors.
Combination with other medicinal products
Co-administration of esomeprazole with atazanavir is not recommended. If the combination of atazanavir with a proton pump inhibitor is judged unavoidable, close clinical monitoring is recommended in combination with an increase in the dose of atazanavir to 400 mg with 100 mg of ritonavir; esomeprazole 20 mg should not be exceeded. Esomeprazole is a CYP2C19 inhibitor. When starting or ending treatment with esomeprazole, the potential for interactions with medicinal products metabolised through CYP2C19 should be considered. An interaction is observed between clopidogrel and esomeprazole. The clinical relevance of this interaction is uncertain. As a precaution, concomitant use of esomeprazole and clopidogrel should be discouraged.
When prescribing esomeprazole for on demand therapy, the implications for interactions with other pharmaceuticals, due to fluctuating plasma concentrations of esomeprazole should be considered.
Serious cutaneous adverse reactions (SCARs)
Serious cutaneous adverse reactions (SCARs) such as erythema multiforme (EM), StevensJohnson syndrome (SJS), toxic epidermal necrolysis (TEN) and drug reaction with eosinophilia and systemic symptoms (DRESS), which can be life-threatening, have been reported very rarely in association with esomeprazole treatment. Patients should be advised of the signs and symptoms of the severe skin reaction EM/SJS/TEN/DRESS and should seek medical advice from their physician immediately when observing any indicative signs or symptoms. Esomeprazole should be discontinued immediately upon signs and symptoms of severe skin reactions and additional medical care/close monitoring should be provided as needed. Rechallenge should not be undertaken in patients with EM/SJS/TEN/DRESS.
Interference with laboratory tests
Increased Chromogranin A (CgA) level may interfere with investigations for neuroendocrine tumours. To avoid this interference, esomeprazole treatment should be stopped for at least 5 days before CgA measurements
4.5 Drug Interactions
Effects of esomeprazole on the pharmacokinetics of other medicinal products
Protease inhibitors
Omeprazole has been reported to interact with some protease inhibitors. The clinical importance and the mechanisms behind these reported interactions are not always known. Increased gastric pH during omeprazole treatment may change the absorption of the protease inhibitors. Other possible interaction mechanisms are via inhibition of CYP2C19.
For atazanavir and nelfinavir, decreased serum levels have been reported when given together with omeprazole and concomitant administration is not recommended. Co-administration of omeprazole (40 mg once daily) with atazanavir 300 mg/ritonavir 100 mg to healthy volunteers resulted in a substantial reduction in atazanavir exposure (approximately 75% decrease in AUC, C max and C min ). Increasing the atazanavir dose to 400 mg did not compensate for the impact of max min omeprazole on atazanavir exposure. The co-administration of omeprazole (20 mg qd) with atazanavir 400 mg/ritonavir 100 mg to healthy volunteers resulted in a decrease of approximately 30% in the atazanavir exposure as compared with the exposure observed with atazanavir 300 mg/ritonavir 100 mg qd without omeprazole 20 mg qd. Co-administration of omeprazole (40 mg qd) reduced mean nelfinavir AUC, Cmax and Cmin by 36-39% and mean AUC, Cmax and Cmin for the pharmacologically active metabolite M8 was reduced by 75-92%. Due to the similar pharmacodynamic effects and pharmacokinetic properties of omeprazole and esomeprazole, concomitant administration with esomeprazole and atazanavir is not recommended and concomitant administration with esomeprazole and nelfinavir is contraindicated. For saquinavir (with concomitant ritonavir), increased serum levels (80-100%) have been reported during concomitant omeprazole treatment (40 mg qd). Treatment with omeprazole 20 mg qd had no effect on the exposure of darunavir (with concomitant ritonavir) and amprenavir (with concomitant ritonavir). Treatment with esomeprazole 20 mg qd had no effect on the exposure of amprenavir (with and without concomitant ritonavir). Treatment with omeprazole 40 mg qd had no effect on the exposure of lopinavir (with concomitant ritonavir)
Methotrexate
When given together with PPIs, methotrexate levels have been reported to increase in some patients. In high-dose methotrexate administration a temporary withdrawal of esomeprazole may need to be considered.
Tacrolimus
Concomitant administration of esomeprazole has been reported to increase the serum levels of tacrolimus. A reinforced monitoring of tacrolimus concentrations as well as renal function (creatinine clearance) should be performed, and dosage of tacrolimus adjusted if needed.
Medicinal products with pH dependent absorption
Gastric acid suppression during treatment with esomeprazole and other PPIs might decrease or increase the absorption of medicinal products with a gastric pH dependent absorption. As with other medicinal products that decrease intragastric acidity, the absorption of medicinal products such as ketoconazole, itraconazole and erlotinib can decrease and the absorption of digoxin can increase during treatment with esomeprazole. Concomitant treatment with omeprazole (20 mg daily) and digoxin in healthy subjects increased the bioavailability of digoxin by 10% (up to 30% in two out of ten subjects). Digoxin toxicity has been rarely reported. However, caution should be exercised when esomeprazole is given at high doses in elderly patients. Therapeutic drug monitoring of digoxin should then be reinforced.
Medicinal products metabolised by CYP2C19
Esomeprazole inhibits CYP2C19, the major esomeprazole metabolising enzyme. Thus, when esomeprazole is combined with medicinal products metabolised by CYP2C19, such as diazepam, citalopram, imipramine, clomipramine, phenytoin etc., the plasma concentrations of these medicinal products may be increased and a dose reduction could be needed. This should be considered especially when prescribing esomeprazole for on demand therapy
Diazepam
Concomitant administration of 30 mg esomeprazole resulted in a 45% decrease in clearance of the CYP2C19 substrate diazepam.
Phenytoin
Concomitant administration of 40 mg esomeprazole resulted in a 13% increase in trough plasma levels of phenytoin in epileptic patients. It is recommended to monitor the plasma concentrations of phenytoin when treatment with esomeprazole is introduced or withdrawn.
Voriconazole
Omeprazole (40 mg once daily) increased voriconazole (a CYP2C19 substrate) Cmax and AUC by 15% and 41%, respectively.
Cilostazol
Omeprazole as well as esomeprazole act as inhibitors of CYP2C19. Omeprazole, given in doses of 40 mg to healthy subjects in a cross-over study, increased C and AUC for cilostazol by 18% and max 26% respectively, and one of its active metabolites by 29% and 69% respectively.
Cisapride
In healthy volunteers, concomitant administration of 40 mg esomeprazole and cisapride resulted in a 32% increase in area under the plasma concentration-time curve (AUC) and a 31% prolongation of elimination half-life (t ) but no significant increase in peak plasma levels of cisapride. The slightly 1/2 prolonged QTc interval observed after administration of cisapride alone, was not further prolonged when cisapride was given in combination with esomeprazole.
Warfarin
Concomitant administration of 40 mg esomeprazole to warfarin-treated patients in a clinical trial showed that coagulation times were within the accepted range. However, post-marketing, a few isolated cases of elevated INR of clinical significance have been reported during concomitant treatment. Monitoring is recommended when initiating and ending concomitant esomeprazole treatment, during treatment with warfarin or other coumarine derivatives.
Clopidogrel
Results from studies in healthy subjects have shown a pharmacokinetic (PK)/ pharmacodynamic (PD) interaction between clopidogrel (300 mg loading dose/75 mg daily maintenance dose) and esomeprazole (40 mg p.o.daily) resulting in decreased exposure to the active metabolite of clopidogrel by an average of 40% and resulting in decreased maximum inhibition of (ADP induced) platelet aggregation by an average of 14%. When clopidogrel was given together with a fixed dose combination of esomeprazole 20 mg +ASA 81 mg compared to clopidogrel alone in a study in healthy subjects there was a decreased exposure by almost 40% of the active metabolite of clopidogrel. However, the maximum levels of inhibition of (ADP induced) platelet aggregation in these subjects were the same in the clopidogrel and the clopidogrel + the combined (esomeprazole + ASA) product groups. Inconsistent data on the clinical implications of a PK/PD interaction of esomeprazole in terms of major cardiovascular events have been reported from both observational and clinical studies. As a precaution concomitant use of clopidogrel should be discouraged.
Investigated medicinal products with no clinically relevant interaction
Amoxicillin and quinidine
Esomeprazole has been shown to have no clinically relevant effects on the pharmacokinetics of amoxicillin or quinidine
Naproxen or rofecoxib
Studies evaluating concomitant administration of esomeprazole and either naproxen or rofecoxib did not identify any clinically relevant pharmacokinetic interactions during short-term studies.
Effects of other medicinal products on the pharmacokinetics of esomeprazole Medicinal products which inhibit CYP2C19 and/or CYP3A4
Esomeprazole is metabolised by CYP2C19 and CYP3A4. Concomitant administration of esomeprazole and a CYP3A4 inhibitor, clarithromycin (500 mg b.i.d.), resulted in a doubling of the exposure (AUC) to esomeprazole. Concomitant administration of esomeprazole and a combined inhibitor of CYP2C19 and CYP 3A4 may result in more than doubling of the esomeprazole exposure. The CYP2C19 and CYP3A4 inhibitor voriconazole increased omeprazole AUCt by 280%. A dose adjustment of esomeprazole is not regularly required in either of these situations. However, dose adjustment should be considered in patients with severe hepatic impairment and if long-term treatment is indicated. Long-term treatment is indicated in adults and adolescents (12 years and older).
Medicinal products which induce CYP2C19 and/or CYP3A4
Medicinal products known to induce CYP2C19 or CYP3A4 or both (such as rifampicin and St. John's wort) may lead to decreased esomeprazole serum levels by increasing the esomeprazole metabolism.
Paediatric population
Interaction studies have only been performed in adults.
4.6 Special populations
Pregnancy
Clinical data on exposed pregnancies with Zosa Junior are insufficient. With the racemic mixture omeprazole, data on a larger number of exposed pregnancies from epidemiological studies indicate no malformative nor foetotoxic effect. Animal studies with esomeprazole do not indicate direct or indirect harmful effects with respect to embryonal/foetal development. Animal studies with the racemic mixture do not indicate direct or indirect harmful effects with respect to pregnancy, parturition or postnatal development. Caution should be exercised when prescribing to pregnant women. A moderate amount of data on pregnant women (between 300-1000 pregnancy outcomes) indicates no malformative or foeto/neonatal toxicity of esomeprazole. Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity.
Breast-feeding
It is not known whether esomeprazole is excreted in human breast milk. There is insufficient information on the effects of esomeprazole in newborns/infants. Esomeprazole should not be used during breast-feeding
Fertility
Animal studies with the racemic mixture omeprazole, given by oral administration do not indicate effects with respect to fertility.
4.7 Effects on ability to drive and use machines
Esomeprazole has minor influence on the ability to drive and use machines. Adverse reactions such as dizziness (uncommon) and blurred vision (rare) has been reported. If affected patients should not drive or use machines.
4.8 Undesirable Effects
Headache, abdominal pain, diarrhoea and nausea are among those adverse reactions that have been most commonly reported.
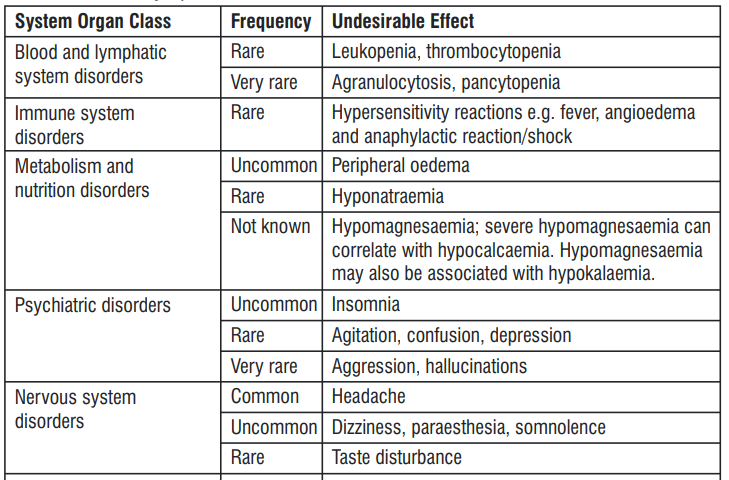
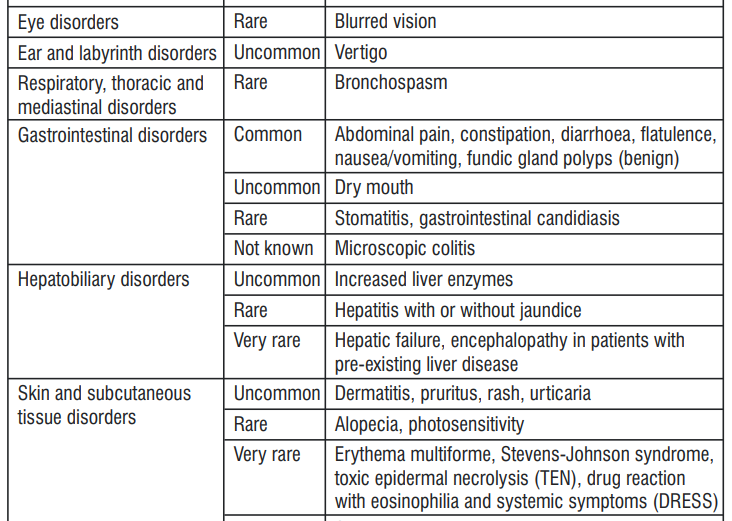
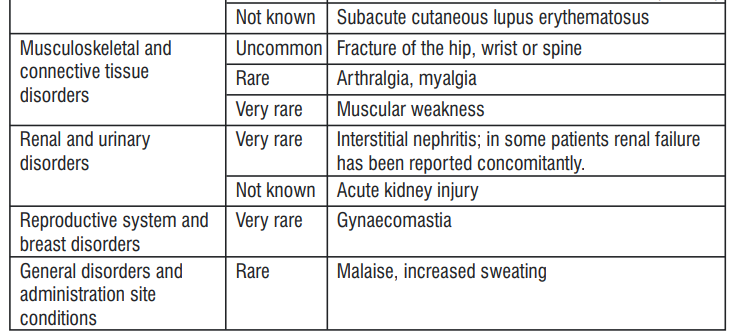
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com Website: https://www.zuventus.com/drug-safety-reporting By reporting side effects, you can help provide more information on the safety of this medicine.
4.9: Overdose
There is very limited experience to date with deliberate overdose. The symptoms described in connection with 280 mg were gastrointestinal symptoms and weakness. Single doses of 80 mg esomeprazole were uneventful. No specific antidote is known. Esomeprazole is extensively plasma protein bound and is therefore not readily dialyzable. As in any case of overdose, treatment should be symptomatic and general supportive measures should be utilised.
5.0 Pharmacological properties
5.1 Mechanism of Action
Esomeprazole is a weak base and is concentrated and converted to the active form in the highly acidic environment of the secretory canaliculi of the parietal cell, where it inhibits the enzyme H+K + -ATPase – the acid pump and inhibits both basal and stimulated acid secretion.
5.2 Pharmacodynamic Properties
After oral dosing with esomeprazole 20 mg and 40 mg the onset of effect occurs within one hour. After repeated administration with 20 mg esomeprazole once daily for five days, mean peak acid output after pentagastrin stimulation is decreased 90% when measured 6 – 7 hours after dosing on day five. After five days of oral dosing with 20 mg and 40 mg of esomeprazole, intragastric pH above 4 was maintained for a mean time of 13 hours and 17 hours, respectively over 24 hours in symptomatic GERD patients. The proportion of patients maintaining an intragastric pH above 4 for at least 8, 12 and 16 hours respectively were for esomeprazole 20 mg 76%, 54% and 24%. Corresponding proportions for esomeprazole 40 mg were 97%, 92% and 56%. Using AUC as a surrogate parameter for plasma concentration, a relationship between inhibition of acid secretion and exposure has been shown. Healing of reflux esophagitis with esomeprazole 40 mg occurs in approximately 78% of patients after four weeks, and in 93% after eight weeks. During treatment with antisecretory medicinal products, serum gastrin increases in response to the decreased acid secretion. Also CgA increases due to decreased gastric acidity. The increased CgA level may interfere with investigations for neuroendocrine tumours. Available published evidence suggests that proton pump inhibitors should be discontinued between 5 days and 2 weeks prior to CgA measurements. This is to allow CgA levels that might be spuriously elevated following PPI treatment to return to reference range. An increased number of ECL cells possibly related to the increased serum gastrin levels, have been observed in both children and adults during long term treatment with esomeprazole. The findings are considered to be of no clinical significance. During long-term treatment with antisecretory medicinal products gastric glandular cysts have been reported to occur at a somewhat increased frequency. These changes are a physiological consequence of pronounced inhibition of acid secretion, are benign and appear to be reversible. Decreased gastric acidity due to any means including proton pump inhibitors, increases gastric count of bacteria normally present in the gastrointestinal tract. Treatment with proton pump inhibitors may lead to a slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter and, in hospitalised patients, possibly also Clostridium difficile.
5.3 Pharmacokinetic properties
Absorption
Esomeprazole is acid labile and is administered orally as enteric-coated granules. In vivo conversion to the R-isomer is negligible. Absorption of esomeprazole is rapid, with peak plasma levels occurring approximately 1-2 hours after dose. The absolute bioavailability is 64% after a single dose of 40 mg and increases to 89% after repeated once-daily administration. For 20 mg esomeprazole the corresponding values are 50% and 68%, respectively. Food intake both delays and decreases the absorption of esomeprazole although this has no significant influence on the effect of esomeprazole on intragastric acidity.
Distribution
The apparent volume of distribution at steady state in healthy subjects is approximately 0.22 L/kg body weight. Esomeprazole is 97% plasma protein bound.
Biotransformation
Esomeprazole is completely metabolised by the cytochrome P450 system (CYP). The major part of the metabolism of esomeprazole is dependent on the polymorphic CYP2C19, responsible for the formation of the hydroxy- and desmethyl metabolites of esomeprazole. The remaining part is dependent on another specific isoform, CYP3A4, responsible for the formation of esomeprazole sulphone, the main metabolite in plasma.
Elimination
The parameters below reflect mainly the pharmacokinetics in individuals with a functional CYP2C19 enzyme, extensive metabolisers. Total plasma clearance is about 17 L/h after a single dose and about 9 L/h after repeated administration. The plasma elimination half-life is about 1.3 hours after repeated once-daily dosing. Esomeprazole is completely eliminated from plasma between doses with no tendency for accumulation during once-daily administration. The major metabolites of esomeprazole have no effect on gastric acid secretion. Almost 80% of an oral dose of esomeprazole is excreted as metabolites in the urine, the remainder in the faeces. Less than 1% of the parent drug is found in urine
6.0 Nonclinical Properties
6.1 Animal Toxicology or Pharmacology
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development. Adverse reactions not observed in clinical studies, but seen in animals at exposure levels similar to clinical exposure levels and with possible relevance to clinical use were as follows: Carcinogenicity studies in the rat with the racemic mixture have shown gastric ECL-cell hyperplasia and carcinoids. These gastric effects in the rat are the result of sustained, pronounced hypergastrinaemia secondary to reduced production of gastric acid and are observed after longterm treatment in the rat with inhibitors of gastric acid secretion. No new or unexpected toxicity findings were observed in juvenile rats and dogs, after administration of esomeprazole for up to 3 months, as compared to the adult animals.
7.0 Description
The active ingredient in Zosa Junior (esomeprazole magnesium trihydrate) granules is bis(5- methoxy-2sulfinyl]-1H-benzimidazole-1-yl) magnesium trihydrate. Esomeprazole is the S-isomer of omeprazole, which is a mixture of the S-and R-isomers. Its molecular formula is (C12H18N3O3S)2 Mg x 3H2O with molecular weight of 767.2 as a trihydrate.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not Applicable.
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
A sachet of 1g.
8.4 Storage and handing instructions
Store protected from light & moisture, at a temperature not exceeding 30°C.
9.0 Patient counselling information
What is ZOSA JUNIOR and what is it used for?
ZOSA JUNIOR contains a medicine called esomeprazole. This belongs to a group of medicines called 'proton pump inhibitors'. They work by reducing the amount of acid that your stomach produces.
It is used to treat the following conditions:
- Treatment of Gastroesophageal Reflux Disease (GERD).
- Risk Reduction of NSAID Associated Gastric Ulcer.
- H. Pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence.
- Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
What you need to know before you take esomeprazole?
Do not take esomeprazole
- If you are allergic to esomeprazole or any of the other ingredients of this medicine.
- If you are allergic to other proton pump inhibitor medicines (e.g. pantoprazole, lansoprazole, rabeprazole, omeprazole).
- If you are taking a medicine containing nelfinavir (used to treat HIV infection). You must not take esomeprazole if any of the above apply to you. If you are not sure, talk to your doctor or nurse before you are given this medicine.
Warnings and precautions
Talk to your doctor or nurse before you are given esomeprazole if:
- You have severe liver problems.
- You have severe kidney problems.
- You have ever had a skin reaction after treatment with a medicine similar to Esomeprazole that reduces stomach acid.
- You are due to have a specific blood test (Chromogranin A). Esomeprazole may hide the symptoms of other diseases. Therefore, if any of the following happen to you before you take esomeprazole or after you are given it, talk to your doctor straight away:
- You lose a lot of weight for no reason and have problems swallowing.
- You get stomach pain or indigestion.
- You begin to vomit food or blood.
- You pass black stools (blood-stained faeces).
If you have been prescribed esomeprazole “on demand” you should contact your doctor if your symptoms continue or change in character. Taking a proton pump inhibitor like esomeprazole, especially over a period of more than one year, may slightly increase your risk of fracture in the hip, wrist, or spine. Tell your doctor if you have osteoporosis or if you are taking corticosteroids (which can increase the risk of osteoporosis). If you get a rash on your skin, especially in areas exposed to the sun tell your doctor as soon as you can, as you may need to stop your treatment with esomeprazole. Remember to also mention any other ill effects like pain in your joints.
Other medicines and esomeprazole Tell your doctor or nurse if you are taking, have recently taken, or might take any other medicines. This includes medicines that you buy without a prescription. This is because esomeprazole can affect the way some medicines work, and some medicines can have an effect on esomeprazole. You must not be given esomeprazole if you are taking a medicine containing nelfinavir (used to treat HIV infection).
Tell your doctor or nurse if you are taking any of the following medicines:
- Atazanavir (used to treat HIV infection).
- Clopidogrel (used to prevent blood clots).
- Ketoconazole, itraconazole or voriconazole (used to treat infections caused by a fungus).
- Erlotinib (used to treat cancer).
- Citalopram, imipramine or clomipramine (used to treat depression).
- Diazepam (used to treat anxiety, relax muscles or in epilepsy).
- Phenytoin (used in epilepsy). If you are taking phenytoin, your doctor will need to monitor you when you start or stop having esomeprazole.
- Medicines that are used to thin your blood, such as warfarin. Your doctor may need to monitor you when you start or stop having esomeprazole.
- Cilostazol (used to treat intermittent claudication – a pain in your legs when you walk which is caused by an insufficient blood supply).
- Cisapride (used for indigestion and heartburn).
- Digoxin (used for heart problems).
- Methotrexate (a chemotherapy medicine used in high doses to treat cancer) – if you are taking a high dose of methotrexate, your doctor may temporarily stop your esomeprazole treatment.
- Tacrolimus (organ transplantation).
- Rifampicin (used for treatment of tuberculosis).
- St. John's wort (Hypericum perforatum) (used to treat depression).
If your doctor has prescribed the antibiotics amoxicillin and clarithromycin as well as esomeprazole to treat ulcers caused by Helicobacter pylori infection, it is very important that you tell your doctor about any other medicines you are taking.
Esomeprazole with food and drink
Esomeprazole should be taken at least 1 hour before meals
Pregnancy, breast-feeding and fertility
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before you are given this medicine. Your doctor will decide whether you can take esomeprazole during this time. It is not known if esomeprazole passes into breast milk. Therefore, you should not be given esomeprazole if you are breastfeeding.
Driving and using machines
Esomeprazole is not likely to affect you being able to drive or use any tools or machines. However, side effects such as dizziness and blurred vision may uncommonly occur. If affected, you should not drive or use machines.
Elderly
Dose adjustment is not required in the elderly
If you take more esomeprazole than you should
If you take more esomeprazole than prescribed by your doctor, talk to your doctor or pharmacist straight away
12.0 Date of issue
13 January 2023
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
- What Zosa Junior is and what it is used for
- What you need to know before you take Zosa Junior
- How to take Zosa Junior
- Possible side effects
- How to store Zosa Junior
- Contents of the pack and other information
1. What Zosa Junior is and what it is used for
Zosa Junior contains a substance called esomeprazole. This belongs to a group of medicines called proton pump inhibitors. These work by reducing the amount of acid that your stomach produces.
Zosa Junior is used to treat the following conditions:
Children over 1 year of age
Zosa Junior is used to treat a condition called “gastroesophageal reflux disease” (GERD).
- This is where acid from the stomach escapes into the gullet (esophagus) causing pain, inflammation and heartburn. Heartburn is a burning feeling rising from the stomach or lower chest up towards the neck.
- In children, the symptoms of the condition can include the return of stomach contents into the mouth (regurgitation), being sick (vomiting) and poor weight gain.
- Children over 4 years of age
- Ulcers which are infected with bacteria called ‘Helicobacter pylori’. If your child has this condition, your doctor may also prescribe antibiotics to treat the infection and allow the ulcer to heal.
2. What you need to know before you take Zosa Junior
Do not take Zosa Junior:
- If you are allergic to esomeprazole or other similar proton pump inhibitors (e.g. pantoprazole, lansoprazole, rabeprazole, omeprazole), or any other ingredients of this medicine (listed in section 6).
- If you are taking a medicine containing nelfinavir (used to treat HIV infection).
Do not take Zosa Junior if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before taking Zosa Junior.
Warnings and precautions
Talk to your doctor or pharmacist before taking Zosa Junior:
- If you have severe liver problems.
- If you have severe kidney problems.
- If you have ever had a skin reaction after treatment with a medicine similar to Zosa Junior that reduces stomach acid.
- If you are due to have a specific blood test (Chromogranin A).
Zosa Junior may hide the symptoms of other diseases. Therefore, if any of the following happen to you while you are taking Zosa Junior, you should talk to your doctor immediately:
- You lose a lot of weight for no reason.
- You get stomach pain or indigestion.
- You begin to vomit repeatedly.
- You have problems swallowing.
- You vomit blood or pass black (blood-stained) motions (faeces).
If you have been prescribed Zosa Junior “on demand” you should contact your doctor if the symptoms are persistent or change character. “On demand” treatment has not been investigated in children and is therefore not recommended in this patient group.
Taking a proton pump inhibitor like Zosa Junior, especially over a period of more than one year, may slightly increase your risk of fracture in the hip, wrist or spine. Tell your doctor if you have osteoporosis or if you are taking corticosteroids (which can increase the risk of osteoporosis).
Rash and skin symptoms
If you get a rash on your skin, especially in areas exposed to the sun tell your doctor as soon as you can, as you may need to stop your treatment with Zosa Junior. Remember to also mention any other ill-effects like pain in your joints.
Serious skin rashes have occurred in patients taking esomeprazole (see also section 4). The rash can involve ulcers of the mouth, throat, nose, genitals and conjunctivitis (red and swollen eyes). These serious skin rashes often come after flu-like symptoms such as fever, headache, body ache. The rash may cover large parts of the body with blistering and peeling of the skin.
If at any time during the treatment (even after several weeks) you develop a rash or any of these skin symptoms, stop taking this medicine and contact your doctor immediately.
Other medicines and Zosa Junior
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This includes medicines that you buy without a prescription. This is because Zosa Junior can affect the way some medicines work and some medicines can have an effect on Zosa Junior.
Do not take Zosa Junior if you are taking nelfinavir (used to treat HIV infection).
Tell your doctor or pharmacist if you are taking any of the following medicines:
- Atazanavir (used to treat HIV infection).
- Clopidogrel (used to prevent blood clots).
- Ketoconazole, itraconazole or voriconazole (used to treat infections caused by a fungus).
- Erlotinib (used to treat cancer).
- Diazepam (used to treat anxiety or relax muscles).
- Citalopram, imipramine or clomipramine (used to treat depression).
- Phenytoin (used in epilepsy).
- Warfarin or coumarin (medicines called anticoagulants that are used to thin your blood).
- Cilostazol (used to treat intermittent claudication – a pain in your legs when you walk which is caused by an insufficient blood supply).
- Cisapride (used for indigestion and heartburn).
- Digoxin (used for heart problems).
- Methotrexate (a chemotherapy medicine used in high doses to treat cancer)
– if you are taking a high dose of methotrexate, your doctor may temporarily stop your Zosa Junior treatment.
- Tacrolimus (organ transplantation).
- Rifampicin (used for treatment of tuberculosis).
- St. John’s wort (Hypericum perforatum) (used to treat depression).
If your doctor has prescribed the antibiotics amoxicillin and clarithromycin as well as Zosa Junior to treat ulcers caused by Helicobacter pylori infection, it is very important that you tell your doctor about any other medicines you are taking.
Zosa Junior gastro-resistant granules with food and drink
Zosa Junior gastro-resistant granules can be taken with or without food.
Pregnancy, breast-feeding and fertility
If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Your doctor will decide whether you can take Zosa Junior during this time.
It is not known if Zosa Junior passes into breast milk. Therefore you should not take Zosa Junior if you are breast-feeding.
Driving and using machines
Zosa Junior is not likely to affect you being able to drive or use tools or machines. However, side effects such as dizziness and blurred vision may uncommonly or rarely occur (see section 4). If affected, you should not drive or use machines.
Zosa Junior contains sucrose and glucose
Zosa Junior contains sucrose and glucose which are both types of sugars. Careful oral hygiene and regular tooth brushing are therefore important. If you have been told by your doctor, that you have an intolerance to some sugars, contact your doctor before taking Zosa Junior
3. How to take Zosa Junior
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Your medicine comes as granules in individual sachets. Each sachet contains 10 mg of esomeprazole. Your doctor will tell you how many sachets to take each day. He or she will also tell you how long you should take them for.
- Empty the contents of the sachet or sachets into a glass containing some water. Do not use fizzy (carbonated) water. The amount of water depends on the number of sachets that your doctor has told you to take at one time.
- Use 15 millilitres (ml) of water (3 teaspoonfuls) for each sachet. This means that you will need 15 ml for one sachet and 30 ml for two sachets.
- Stir the granules in the water.
- Leave the mixture for a few minutes until it has thickened.
- Stir again and drink the mixture. The granules must not be chewed or crushed. Do not leave the mixture to stand for more than 30 minutes before you drink it.
- If anything remains in the glass, add some more water, stir and drink it immediately.
Zosa Junior gastro-resistant granules can be taken with or without food.
If you are being fed using a feeding (gastric) tube, your doctor or nurse can give you Zosa Junior through your tube. Information for your doctor or nurse is provided at the end of this leaflet.
The recommended doses are given below:
Use in children aged 1 to 11 years
- Zosa Junior is not recommended for children younger than 1 year.
To treat gastroesophageal reflux disease (GERD)
- The recommended dose is one sachet (10 mg) or two sachets (20 mg) once daily. The dose for children is based on the child’s weight and the doctor will decide the correct dose.
Use in children aged 4 years and older
To treat ulcers caused by Helicobacter pylori infection and to stop them coming back.
- The dose for children is based on the child’s weight and your doctor will decide the correct dose. The doctor will also prescribe two antibiotics for your child.
Use in adults and adolescents
Zosa Junior oral suspension may also be used by patients having difficulty swallowing dispersed Zosa Junior gastro-resistant tablets. Information on dosing for patients from the age of 12 years is in gastro-resistant tablet product information (ask your doctor or pharmacist if you require further information).
Elderly
There is no need to alter the dose if you are elderly.
People with liver problems
- For people with severe liver problems, the maximum daily dose of Zosa Junior is two sachets (20 mg). For children 1-11 years with severe liver problems, a maximum dose of 10 mg should not be exceeded.
People with kidney problems
- There are no special dosage restrictions for people with kidney problems. However, if you have severe kidney problems your doctor may decide to carry out regular tests.
If you take more Zosa Junior than you should
If you have taken more Zosa Junior than prescribed by your doctor, seek medical advice.
If you forget to take Zosa Junior
If you forget to take a dose, take it as soon as you remember. If it is almost time to take the next dose, wait until then. Do not take a double dose to make up for the forgotten dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you notice any of the following serious side effects, stop taking Zosa Junior and contact a doctor immediately:
- Yellow skin, dark urine and tiredness which can be symptoms of liver problems. These effects are rare, and may affect up to 1 in 1,000 people.
- Sudden wheezing, swelling of your lips, tongue and throat or body, rash, fainting or difficulties in swallowing (severe allergic reaction). These effects are rare, and may affect up to 1 in 1,000 people.
- Sudden onset of a severe rash or reddening of the skin with blisters or peeling may occur even after several weeks of treatment. There may also be severe blisters and bleeding in the lips, eyes, mouth, nose and genitals. The skin rashes may develop into serious widespread skin damage (peeling of the epidermis and superficial mucous membranes) with life threatening consequences. This could be ‘erythema multiforme’, ‘Stevens-Johnson syndrome’, ‘toxic epidermal necrolysis’ or ‘drug reaction with eosinophilia and systemic symptoms’. These effects are very rare, and might affect up to 1 in 10,000 people.
Other side effects include:
Common (may affect up to 1 in 10 people)
- Headache.
- Effects on your stomach or gut: diarrhoea, stomach pain, constipation, wind (flatulence).
- Feeling sick (nausea) or being sick (vomiting).
- Benign polyps in the stomach.
Uncommon (may affect up to 1 in 100 people)
- Swelling of the feet and ankles.
- Disturbed sleep (insomnia).
- Dizziness, tingling feelings such as “pins and needles”, feeling sleepy.
- Spinning feeling (vertigo).
- Dry mouth.
- Changes in blood tests that check how the liver is working.
- Skin rash, lumpy rash (hives) and itchy skin.
- Fracture of the hip, wrist or spine (if Zosa Junior is used in high doses and over long duration).
Rare (may affect up to 1 in 1,000 people)
- Blood problems such as a reduced number of white cells or platelets. This can cause weakness, bruising or make infections more likely.
- Low levels of sodium in the blood. This may cause weakness, being sick (vomiting) and cramps.
- Feeling agitated, confused or depressed.
- Taste changes.
- Eyesight problems such as blurred vision.
- Suddenly feeling wheezy or short of breath (bronchospasm).
- An inflammation of the inside of the mouth.
- An infection called “thrush” which can affect the gut and is caused by a fungus.
- Liver problems, including jaundice which can cause yellow skin, dark urine, and tiredness.
- Hair loss (alopecia).
- Skin rash on exposure to sunshine.
- Joint pains (arthralgia) or muscle pains (myalgia).
- Generally feeling unwell and lacking energy.
- Increased sweating.
Very rare (may affect up to 1 in 10,000 people)
- Changes in blood count including agranulocytosis (lack of white blood cells)
- Aggression.
- Seeing, feeling or hearing things that are not there (hallucinations).
- Severe liver problems leading to liver failure and inflammation of the brain.
- Sudden onset of a severe rash or blistering or peeling skin. This may be associated with a high fever and joint pains (Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, , drug reaction with eosinophilia and systemic symptoms).
- Muscle weakness.
- Severe kidney problems.
- Enlarged breasts in men.
Not known (frequency cannot be estimated from the available data)
- If you are on Zosa Junior for more than three months it is possible that the levels of magnesium in your blood may fall. Low levels of magnesium can be seen as fatigue, involuntary muscle contractions, disorientation, convulsions, dizziness or increased heart rate. If you get any of these symptoms, please tell your doctor promptly. Low levels of magnesium can also lead to a reduction in potassium or calcium levels in the blood. Your doctor may decide to perform regular blood tests to monitor your levels of magnesium.
- Inflammation in the gut (leading to diarrhoea).
- Rash, possibly with pain in the joints.
Zosa Junior may in very rare cases affect the white blood cells leading to immune deficiency. If you have an infection with symptoms such as fever with a severely reduced general condition or fever with symptoms of a local infection such as pain in the neck, throat or mouth or difficulties in urinating, you must consult your doctor as soon as possible so that a lack of white blood cells (agranulocytosis) can be ruled out by a blood test. It is important for you to give information about your medication at this time.
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Zosa Junior
This medicinal product does not require any special storage conditions.
- Keep out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and sachet after EXP. The expiry date refers to the last day of that month.
- The reconstituted suspension should be used within 30 minutes.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What Zosa Junior gastro-resistant granules for oral suspension contains
Each sachet contains :
Esomeprazole Magnesium Trihydrate IP
equivalent to Esomeprazole -10 mg
(As Gastro-resistant form)
Colour : Sunset Yellow FC