Zosa L Capsules
Therapy Area
Gastrointestinal
1.0 Name of the medicinal product
Esomeprazole (GR) & Levosulpiride (PR) Capsules
2.0 Qualitative and quantitative composition
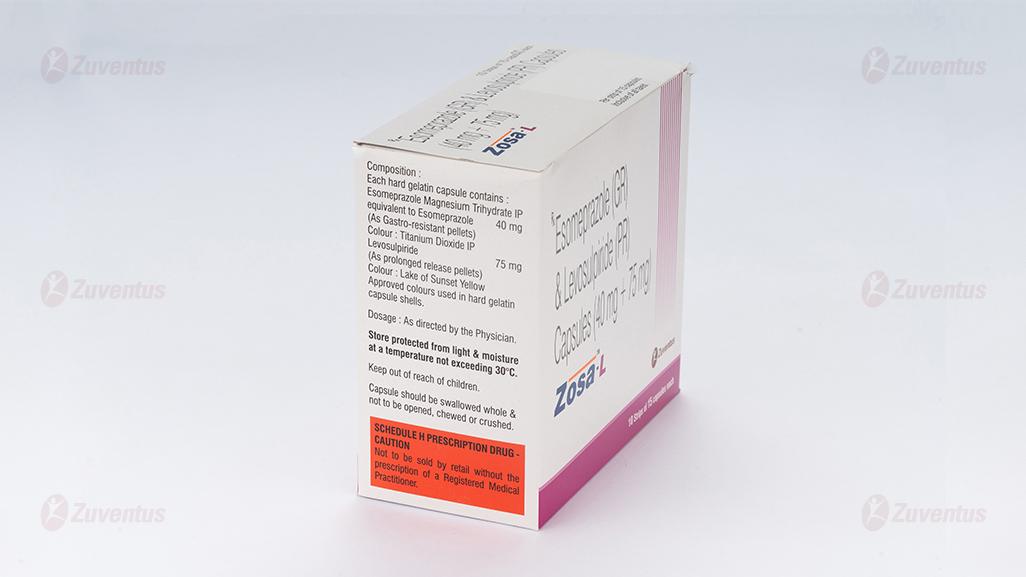
Each Hard Gelatin Capsule Contains:
Esomeprazole Magnesium USP (Trihydrate)
equivalent to Esomeprazole ……. ….. 40 mg
(as gastro-resistant pellets)
Levosulpiride ………………………… 75 mg
(as prolonged release pellets)
3.0 Pharmaceutical form and strength
Capsule Esomeprazole (40 mg) & Levosulpiride (75 mg)
4.0 Clinical particulars
4.1 Therapeutic indications
For the treatment of gastro-esophageal reflux disease (GERD) in patients who do not respond to PPI (proton-pump inhibitor) alone.
4.2 Posology and method of administration
1 capsule to be administered once daily. Zosa L Capsules should be administered on empty stomach, preferably in the morning or at least 1 hour prior to meal. The capsules should be swallowed whole with water and not to be opened, chewed or crushed. Or, as prescribed by the physician
Pediatric Patients
Safety and efficacy of esomeprazole with levosulpiride combination therapy has not been established in paediatric patients. Thus, Zosa L Capsules are not recommended for use in children and adolescents below 18 years of age.
Geriatric Patients
No dosage adjustment is generally necessary in the elderly patients with normal renal function, but dose should be reduced if there is evidence of renal impairment. Elderly patients are more susceptible to postural hypotension, sedation, and extrapyramidal side effects. Thus, caution should be exercised in the elderly population while on Zosa L therapy.
Renal Impairment Patients
Zosa L Capsules should be used with caution and dose/dosage frequency may need to be reduced depending on the severity of the renal dysfunction. Zosa L Capsules are contraindicated in patients with severe renal impairment.
Hepatic Impairment Patients
With esomeprazole, dose adjustment is not required in patients with mild to moderate liver impairment. For patients with severe liver impairment, a maximum dose of 20 mg esomeprazole should not be exceeded. There is no information available on use of levosulpiride in patients with hepatic dysfunction. Thus, as a precautionary measure, Zosa L Capsules should be avoided in patients with hepatic impairment.
4.3 Contraindications
- Patients with known hypersensitivity to esomeprazole or to any substituted benzimidazole derivative or to levosulpiride or to any component of the formulation.
- In patients receiving rilpivirine-containing products.
- Gastrointestinal bleeding and intestinal obstruction.
- Severe renal or hepatic insufficiency.
- Porphyrias.
- Alcohol intoxication.
- Certain tumors like phaeochromocytoma and pituitary prolactinoma.
- Concurrent use with levodopa or other antiparkinson drugs (including ropinirole).
4.4 Special warnings and precautions for use
Esomeprazole
Gastric Malignancy: In the presence of any alarm symptom (e.g., significant unintentional weight loss, recurrent vomiting, dysphagia, haematemesis or melaena) and when gastric ulcer is suspected or present, malignancy should be excluded, as treatment with esomeprazole may alleviate symptoms and delay diagnosis.
Helicobacter Pylori Eradication: When prescribing esomeprazole for eradication of Helicobacter pylori, possible drug interactions for all components in the triple therapy should be considered. Clarithromycin is a potent inhibitor of CYP3A4 and hence contraindications and interactions for clarithromycin should be considered when the triple therapy is used in patients concurrently taking other drugs metabolised via CYP3A4, such as cisapride.
Gastrointestinal Infections/Gastritis: Treatment with proton pump inhibitors (PPIs) may lead to a slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter. Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole, of which esomeprazole is an enantiomer.
Clostridium Difficile-Associated Diarrhea (CDAD): Published observational studies suggest that PPI therapy like esomeprazole may be associated with an increased risk of Clostridium difficile-associated diarrhea, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve. Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
Absorption of Vitamin B12: Esomeprazole, like all acid-blocking medicines, may reduce the absorption of vitamin B12 (cyanocobalamin) due to hypo- or achlorhydria. This should be considered in patients with reduced body stores or risk factors for reduced vitamin B12 absorption on long-term therapy.
Risk of Bone Fracture: Several published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose (defined as multiple daily doses), and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to established treatment guidelines and they should have an adequate intake of vitamin D and calcium.
Subacute Cutaneous Lupus Erythematosus (SCLE): PPIs have been associated with cases of SCLE, although very infrequently. If lesions occur, especially in sun-exposed areas of the skin, and if accompanied by arthralgia, the patient should seek medical help promptly and esomeprazole therapy should be stopped immediately. The occurrence of SCLE with previous PPI treatment may increase the risk of SCLE with other PPIs.
Hypomagnesemia: Hypomagnesemia (symptomatic/asymptomatic), has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI. For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), it is recommended to monitor magnesium levels prior to initiation of PPI treatment, and periodically thereafter.
Levosulpiride
History of Breast Cancer: Levosulpiride may increase prolactin levels. Therefore, caution should be exercised and patients with a history or a family history of breast cancer should be closely monitored during levosulpiride therapy.
Prolongation of the QT Interval: Levosulpiride induces a prolongation of the QT interval. This effect is known to potentiate the risk of serious ventricular arrhythmias such as torsade de pointes. Levosulpiride should be used with caution in patients with cardiovascular disease or with a family history of QT prolongation.
Gastrointestinal Disorders: Levosulpiride should not be used when gastrointestinal stimulation of motility can be harmful e.g., in presence of gastrointestinal hemorrhage, mechanical obstructions or perforations.
Drugs Acting on CNS: Caution is advised when levosulpiride is administered concomitantly with other centrally acting drugs.
Alcohol: Concomitant intake of alcohol should be avoided during levosulpiride therapy as there is an increased chance of sedation.
Smoking: Smoking increases metabolism of the drug and thus, require higher dose of levosulpiride.
Parkinson’s Disease: In patient with Parkinson's disease, levosulpiride use should be avoided and an alternative drug therapy should be considered.
Convulsions: Cases of convulsions, sometimes in patients with no previous history, have been reported. In patients requiring levosulpiride who are receiving anticonvulsant therapy, the dose of the anticonvulsant should not be changed.
Anticholinergic Effects: Levosulpiride has an anticholinergic effect and, therefore, should be used with caution in patients with a history of glaucoma, ileus, congenital digestive stenosis, urine retention or hyperplasia of the prostate.
Hypertensive Patients: Levosulpiride should be used with caution in hypertensive patients, especially in the elderly population, due to the risk of hypertensive crisis. Patients should be adequately monitored.
4.5 Interaction with other medicinal products and other forms of interaction Esomeprazole
1) Interference with Antiretroviral Therapy Reduced Concentrations of Atazanavir and Nelfinavir: Concomitant use of atazanavir and nelfinavir with PPIs is not recommended. Co-administration of atazanavir with PPIs is expected to substantially decrease atazanavir plasma concentrations and may result in a loss of therapeutic effect and the development of drug resistance. If the combination of atazanavir with a PPI is unavoidable, close clinical monitoring is recommended in combination with an increase in the dose of atazanavir to 400 mg with 100 mg of ritonavir; esomeprazole 20 mg should not be exceeded.
Increased Concentrations of Saquinavir: Co-administration of saquinavir with PPIs is expected to increase saquinavir concentrations, which may increase toxicity and require dose reduction. Omeprazole, of which esomeprazole is an enantiomer, has been reported to interact with other antiretroviral drugs, too. The clinical importance and the mechanisms behind these interactions are not always known. Increased gastric pH during omeprazole treatment may change the absorption of the antiretroviral drug. Other possible interaction mechanisms are via CYP 2C19.
2) Drugs for Which Gastric PH Can Affect Bioavailability (ketoconazole, atazanavir, iron salts, erlotinib, mycophenolate mofetil, digoxin) Esomeprazole inhibits gastric acid secretion. Therefore, esomeprazole may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability. Like with other drugs that decrease intragastric acidity, the absorption of drugs such as ketoconazole, atazanavir, iron salts, erlotinib, and mycophenolate mofetil (MMF) can decrease, while the absorption of drugs such as digoxin can increase during treatment with esomeprazole. Digoxin: Concomitant treatment with omeprazole (20 mg daily), of which esomeprazole is an enantiomer, and digoxin in healthy subjects increased the bioavailability of digoxin by 10%. Co-administration of digoxin with esomeprazole is expected to increase the systemic exposure of digoxin. Therefore, patients may need to be monitored when digoxin is taken concomitantly with esomeprazole.
Mycophenolate Mofetil (MMF): Co-administration of omeprazole in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving esomeprazole and MMF. Use esomeprazole with caution in transplant patients receiving MMF.
3) Effects on Hepatic Metabolism/Cytochrome P-450 Pathways Esomeprazole is extensively metabolized in the liver by CYP 2C19 and CYP 3A4. In-vitro and in-vivo studies have shown that esomeprazole is not likely to inhibit CYPs 1A2, 2A6, 2C9, 2D6, 2E1, and 3A4. No clinically relevant interactions with drugs metabolized by these CYP enzymes would be expected. Drug interaction studies have shown that esomeprazole does not have any clinically significant interactions with quinidine, clarithromycin, or amoxicillin.
Warfarin: Patients treated with PPIs and warfarin concomitantly may need to be monitored for increases in INR (International Normalized Ratio) and prothrombin time. Increases in INR and prothrombin time may lead to abnormal bleeding.
Clopidogrel: Avoid concomitant use of esomeprazole with clopidogrel. Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by use of concomitant medications, such as esomeprazole, that inhibit CYP2C19 activity. Concomitant use of clopidogrel with esomeprazole 40 mg reduces the pharmacological activity of clopidogrel. As a precaution, concomitant use of esomeprazole and clopidogrel should be discouraged or when using esomeprazole consider alternative anti-platelet therapy.
Diazepam: Esomeprazole may potentially interfere with CYP2C19, the major esomeprazole metabolizing enzyme. Co-administration of esomeprazole and diazepam, a CYP2C19 substrate, resulted in a 45% decrease in clearance of diazepam.
Phenytoin: Concomitant administration of esomeprazole 40 mg resulted in a 13% increase in trough plasma levels of phenytoin in epileptic patients. It is recommended to monitor the plasma concentrations of phenytoin when treatment with esomeprazole is introduced or withdrawn.
Cilostazol: Omeprazole as well as esomeprazole act as inhibitors of CYP2C19. Omeprazole, of which esomeprazole is an enantiomer, given in doses of 40 mg to healthy subjects in a cross-over study, increased Cmax and AUC for cilostazol by 18% and 26% respectively, and one of its active metabolites by 29% and 69%, respectively. Cisapride: In healthy volunteers, concomitant administration of esomeprazole 40 mg resulted in a 32% increase in area under the plasma concentration-time curve (AUC) and a 31% prolongation of elimination half-life (t½), but no significant increase in peak plasma levels of cisapride. The slightly prolonged QTc interval observed after administration of cisapride alone, was not further prolonged when cisapride was given in combination with esomeprazole. Voriconazole: Concomitant administration of esomeprazole and a combined inhibitor of CYP 2C19 and CYP3A4, such as voriconazole, may result in a more than doubling of the esomeprazole exposure. Dose adjustment of esomeprazole is not normally required. However, in patients with Zollinger-Ellison's Syndrome, who may require higher doses (up to 240 mg/day), dose adjustment may be considered. Rifampicin: Drugs known to induce CYP2C19 or CYP3A4 or both (such as rifampin) may lead to decreased esomeprazole serum levels. Avoid concomitant use of rifampin with esomeprazole. St. John's Wort: Omeprazole, of which esomeprazole is an enantiomer, has been reported to interact with St. John's wort, an inducer of CYP3A4. Avoid concomitant use of St. John's wort with esomeprazole.
4) Concomitant Administration with Other Drugs
Tacrolimus: Concomitant administration of esomeprazole and tacrolimus may increase the serum levels of tacrolimus.
Combination Therapy with Clarithromycin: Co-administration of esomeprazole, clarithromycin, and amoxicillin has resulted in an increase in plasma levels of esomeprazole and 14-hydroxyclarithromycin.
Methotrexate: Concomitant administration of PPIs and methotrexate (primarily at high dose) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate, leading to a risk of methotrexate toxicity. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients.
5) Drug / Laboratory Tests Interactions
Interactions with Investigations of Neuroendocrine Tumors: Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. To avoid this interference, esomeprazole treatment should be stopped for at least 5 days before CgA measurements; consider repeating the test if initial CgA levels are high. Levosulpiride
Antacids and Sucralfate: Bioavailability of levosulpiride is reduced if it is taken concomitantly with sucralfate and aluminum/magnesium-containing antacids. So, these medicines should not be taken along with levosulpiride. There should be a minimum 2 hour time lag between the two medicines. Anticholinergic Drugs, Narcotics and
Analgesic Drugs: The effect of levosulpiride on gastrointestinal motility can be antagonized by these drugs. Antihypertensive Drugs: Concomitant use of levosulpiride may enhance the hypotensive effects of these drugs.
Anticholinergic Drugs: Concomitant administration may cause increase in incidence of anticholinergic side effects.
Levodopa/Antiparkinson Drugs (including ropinirole): There is reciprocal antagonism of effects between levodopa or antiparkinson drugs (including ropinirole) and levosulpiride. Levodopa reduces effects of levosulpiride; conversely, levosulpiride may decrease the efficacy of levodopa in the management of Parkinson’s disease. Thus, concomitant use of these drugs are contraindicated.
Atomoxetine, Antiarrhythmics, Terfenadine, Chloroquine, Quinine, Cisapride, and Drugs Causing Hypokalemia (corticosteroids, laxatives, and diuretics like furosemide): Concurrent use of levosulpiride with these drugs may cause arrhythmia, especially prolonged QT interval.
Alcohol: Levosulpiride can potentiate the cognitive and motor effects of alcohol. Thus, concurrent use should be avoided.
Lithium: Increased risk of extrapyramidal effects. Discontinuation of both drugs is recommended at first signs of neurotoxicity.
4.6 Use in special populations
Pregnancy Zosa L capsules are not recommended for use in pregnant women. Breast-feeding Zosa L capsules should not be used during breast feeding. Accordingly, a decision should be made whether to discontinue nursing or to discontinue/abstain from drug therapy, taking into account the benefit of the drug to the mother.
4.7 Effects on ability to drive and use machines
Patients should avoid driving or operating machinery or not to engage in activities that require full mental alertness.
4.8 Undesirable effects
Esomeprazole
Acute kidney injury as an adverse drug reaction reported with the use of proton pump inhibitors.
The most frequently reported (≥ 1%) adverse reactions with esomeprazole were headache, diarrhea, nausea, flatulence, abdominal pain, constipation, and dry mouth. Additional adverse reactions that were reported as possibly or probably related to esomeprazole with an incidence < 1% are as follows:
Body as a Whole: Enlarged abdomen, allergic reaction, asthenia, back pain, chest pain, substernal chest pain, facial edema, peripheral edema, hot flushes, fatigue, fever, flu-like symptoms, generalized edema, leg edema, malaise, pain, rigors.
Cardiovascular: Flushing, hypertension, tachycardia.
Endocrine: Goiter.
Gastrointestinal (GI): Bowel irregularity, constipation aggravated, dyspepsia, dysphagia, GI dysplasia, epigastric pain, eructation, esophageal disorders, frequent stools, gastroenteritis, GI hemorrhage, GI symptoms not otherwise specified, hiccup, melena, mouth disorders, pharyngeal disorders, rectal disorders, increase in serum gastrin, tongue disorders, tongue edema, ulcerative stomatitis, vomiting.
Hearing: Earache, tinnitus.
Hematologic: Anemia, cervical lymphadenopathy, epistaxis, leukocytosis, leukopenia, thrombocytopenia.
Hepatic: Abnormalities in hepatic function, including bilirubinemia, increase in AST (aspartate aminotransferase) and ALT (alanine aminotransferase).
Metabolic/Nutritional: Glycosuria, hyperuricemia, hyponatremia, increased alkaline phosphatase, thirst, vitamin B12 deficiency, weight increase/decrease.
Musculoskeletal: Arthropathy, cramps, fibromyalgia syndrome, hernia, polymyalgia rheumatica.
Nervous System/Psychiatric: Anorexia, apathy, increased appetite, confusion, depression aggravated, dizziness, hypertonia, nervousness, hypoesthesia, impotence, insomnia, migraine, paresthesia, sleep disorder, somnolence, tremor, vertigo, visual field defect.
Reproductive: Dysmenorrhea, menstrual disorders, vaginitis.
Respiratory: Aggravated asthma, coughing, dyspnea, laryngeal edema, pharyngitis, rhinitis, sinusitis.
Skin and Appendages: Acne, angioedema, dermatitis, pruritus, rash, urticaria, sweating.
Special Senses: Otitis media, parosmia, taste loss, taste perversion.
Urogenital: Albuminuria, cystitis, dysuria, fungal infection, hematuria, frequent micturition, moniliasis, polyuria.
Visual: Conjunctivitis, abnormal vision.
Laboratory Abnormalities
The following clinically significant laboratory changes in clinical trials, irrespective of relationship to esomeprazole, were reported in ≤ 1% of patients: Increased creatinine, uric acid, total bilirubin, alkaline phosphatase, ALT, AST, hemoglobin, white blood cell count, platelets, serum gastrin, potassium, sodium, thyroxine and thyroid stimulating hormone. Decreased levels of hemoglobin, white blood cell count, platelets, potassium, sodium, and thyroxine.
Post-Marketing Experience The following adverse reactions have been identified during post-marketing use of esomeprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic: Agranulocytosis, pancytopenia.
Eye: Blurred vision.
Gastrointestinal: Pancreatitis, stomatitis, microscopic colitis.
Hepatobiliary: Hepatitis with or without jaundice, hepatic failure.
Immune System: Anaphylactic reaction/shock.
Infections and Infestations: GI candidiasis, Clostridium difficile-associated diarrhea.
Metabolism and Nutritional Disorders: Hypomagnesemia.
Musculoskeletal and Connective Tissue: Muscular weakness, myalgia, fractures.
Nervous System: Hepatic encephalopathy, taste disturbance.
Psychiatric: Aggression, agitation, depression, hallucination.
Renal and Urinary: Interstitial nephritis.
Reproductive System and Breast: Gynecomastia.
Respiratory, Thoracic, and Mediastinal: Bronchospasm.
Skin and Subcutaneous Tissue: Alopecia, erythema multiforme, hyperhidrosis, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis (sometime fatal), cutaneous lupus erythematosus.
Levosulpiride
The following side effects may occur with the use of levosulpiride: Acute muscular dystonia characterized by abnormal movements (twitching, tremor, etc.) of the hands, leg, tongue and facial muscles.
Sedation or drowsiness (because of decrease in sensory inputs to reticular activating system).
Increase in plasma prolactin levels manifested by breast enlargement (gynecomastia), production of milk (galactorrhea) and stopping of menstrual periods (amenorrhea).
Neuroleptic malignant syndrome (characterized by hyperpyrexia, muscle rigidity, increased myoglobin and creatine kinase).
Akathisia (uncontrollable desire to move about without any anxiety).
Tardive dyskinesia, it occurs late in the therapy and its features include involuntary rhythmical movements of face, mouth and jaw. The reason for tardive dyskinesia is synthesis of newer dopamine receptors which are supersensitive to even a small amount of dopamine. This causes a decrease in cholinergic activity in the striatum followed by decrease in gamma-amino butyric acid (GABA) release. This decreased in inhibitory GABA is responsible for increased involuntary motor activity.
Postural hypotension (because of autonomic blockade), tolerance develops to this effect after some time.
Weight gain. Elevated liver transaminases.
Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
Healthcare professionals are asked to report any suspected adverse reactions via
email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Experience with overdose is limited. There is no specific antidote. Treatment is only symptomatic. Appropriate supportive measures should therefore be instituted, close supervision of vital functions and cardiac monitoring (risk of QT interval prolongation and subsequent ventricular arrhythmias) is recommended until the patient recovers. If severe extrapyramidal symptoms occur anticholinergic drugs should be administrated. Overdose may be treated with alkaline osmotic diuresis and, if necessary, anti-parkinson drugs. Coma needs appropriate nursing, and cardiac monitoring is recommended until the patient recovers.
5.0 Pharmacological properties
5.1 Mechanism of action
Esomeprazole
Esomeprazole is a proton pump inhibitor (PPI) that suppresses gastric acid (hydrochloric acid - HCl) secretion by specific inhibition of the H+/K+-ATPase enzyme system at the secretory surface of the gastric parietal cell. Esomeprazole is a weak base and is concentrated and converted to the active form (i.e., the achiral sulphenamide) in the highly acidic environment of the secretory canaliculi of the parietal cell, where it inhibits the enzyme H+K+-ATPase (the acid/proton pump), and inhibits both basal and stimulated acid secretion. By acting specifically on the proton pump, esomeprazole blocks the final step in acid production, thereby reducing gastric acidity.
Levosulpiride
Prokinetic Effect: Levosulpiride is principally a dopamine D2 antagonist. Dopamine has a direct relaxant effect on the gut by activating muscular D2 receptors. Levosulpiride stimulates gut motility by blocking D2 receptors in lower esophageal sphincter (LES) and stomach. Levosulpiride also acts as an agonist on serotonin 5-HT4 receptors and thus, increases acetylcholine level which leads to increase in GI motility. Antiemetic Effect: Levosulpiride exerts a selective antagonist activity on the D2 receptors on neurons in the CNS (postrema area of 4th ventricle) and thus, produces antiemetic effect. Levosulpiride is also a weak inhibitor of 5-HT3 receptors. Levosulpiride also acts as a moderate agonist at the serotonergic (5-HT4) receptor. This enhances its therapeutic efficacy in gastrointestinal disorders (reduction of nausea and vomiting).
5.2 Pharmacodynamic properties
Esomeprazole
Esomeprazole is the S-isomer of omeprazole. Esomeprazole reduces gastric acid secretion through a specific targeted mechanism of action i.e., inhibition of the acid pump in the gastric parietal cell. Both the R- and S-isomer of omeprazole have similar pharmacodynamic activity.
After oral dosing with esomeprazole 20 mg and 40 mg the onset of effect occurs within one hour. After repeated administration with 20 mg esomeprazole once daily for 5 days, mean peak acid output decreases by 90% when measured 6 to 7 hours after dosing on day five.
After 5 days of oral dosing with 20 mg and 40 mg of esomeprazole, intragastric pH above 4 was maintained for a mean time of 13 hours and 17 hours, respectively over 24 hours in symptomatic GERD patients. The proportion of patients maintaining an intragastric pH above 4 for at least 8, 12 and 16 hours respectively were 76%, 54% and 24% for esomeprazole 20 mg. Corresponding proportions for esomeprazole 40 mg were 97%, 92% and 56%.
Esomeprazole increases the mean fasting gastrin level in a dose-related manner. This increase reached a plateau within two to three months of therapy and returned to baseline levels within four weeks after discontinuation of therapy.
Levosulpiride
Levosulpiride, levo-enantiomer (biologically active form) of sulpiride, is a benzamide derivative. The levo-enantiomer shows better/similar pharmacological actions and lower incidence of toxic effects than both dextro as well as the racemic forms of the drug. Due to its peripheral anti-dopaminergic action, levosulpiride exhibits gastrokinetic/prokinetic, antiemetic, and anti-dyspeptic effects. Levosulpiride act as a modulator of the motor activity of the upper digestive tract. Levosulpiride accelerates gastric emptying and improves gastrointestinal (GI) symptoms such as heart burn, regurgitation, etc. by selectively inhibitingdopaminergic receptors (D2) in the submucosal and myoenteric plexus of the gastrointestinal tract (GIT) and chemoreceptor trigger zone (CTZ) of the central nervous system (CNS).
5.3 Pharmacokinetic Properties
Esomeprazole
Absorption: Zosa L Capsules contains esomeprazole as a gastro-resistant tablet. This is necessary because, like other PPIs, esomeprazole is acid-labile drug. Absorption of esomeprazole therefore, begins only after the tablet leaves the stomach. Esomeprazole is rapidly absorbed after oral administration. Onset of effect occurs within one hour after oral dosing with esomeprazole 20 mg and 40 mg. Peak plasma levels (Cmax) occur approximately 1 to 2 hours (Tmax) after oral dose. The Cmax increases proportionally when the dose is increased, and there is a three-fold increase in the area under the plasma concentration-time curve (AUC) from 20 to 40 mg. At repeated once daily dosing with 40 mg, the systemic bioavailability is approximately 90% compared to 64% after a single dose of 40 mg. Effect of Food: Food intake both delays and decreases the absorption of esomeprazole. Thus, esomeprazole should be administered at least one hour before meal. Distribution: Esomeprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 2 to 20 μmol/l. The apparent volume of distribution at steady state in healthy volunteers is approximately 16 liters. Metabolism: Esomeprazole is extensively metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The metabolites of esomeprazole lack antisecretory activity. The major part of esomeprazole's metabolism is dependent upon the CYP2C19 isoenzyme, which forms the hydroxy and desmethyl metabolites. The remaining amount is dependent on CYP3A4 which forms the sulphone metabolite. Excretion: Total plasma clearance is about 17 l/h after a single dose and about 9 l/h after repeated administration. The plasma elimination half-life is about 1.3 hours after repeated once daily dosing. Esomeprazole is completely eliminated from plasma with no tendency for accumulation during once-daily administration. Approximately 80% of an oral dose of esomeprazole is excreted as inactive metabolites in the urine, and the remainder is found as inactive metabolites in the feces. Less than 1% of parent drug is excreted in the urine.
Levosulpiride
Pharmacokinetics of levosulpiride in sustained release formulation is not available. Conventional formulation of levosulpiride (i.e., immediate release) has following pharmacokinetic properties: Levosulpiride when administered orally exhibited linear pharmacokinetic properties over the dose range of 25 to 100 mg. The bioavailability of levosulpiride when given orally is about 30%. Sulpiride is slowly absorbed from the gastrointestinal tract with peak plasma concentrations are attained 3 to 6 hours after oral dose. After repeated administration, steady state was reached on day 4 of multiple dosing. Sulpiride is about 40% bound to plasma proteins and is reported to have a plasma half-life of about 8 to 9 hours. Levosulpiride is mainly excreted through the renal route.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Esomeprazole
Carcinogenicity: The carcinogenic potential of esomeprazole magnesium was assessed using studies of omeprazole, of which esomeprazole is an enantiomer. In two 24-month oral carcinogenicity studies in rats, omeprazole at daily doses of 1.7 mg/kg/day, 3.4 mg/kg/day, 13.8 mg/kg/day, 44 mg/kg/day, and 140.8 mg/kg/day (about 0.4 to 34 times the human dose of 40 mg/day expressed on a body surface area basis) produced gastric ECL cell carcinoids in a dose-related manner in both male and female rats; the incidence of this effect was markedly higher in female rats, which had higher blood levels of omeprazole. In addition, ECL cell hyperplasia was present in all treated groups of both sexes. In one of these studies, female rats were treated with 13.8 mg omeprazole/kg/day (about 3.4 times the human dose of 40 mg/day on a body surface area basis) for 1 year, then followed for an additional year without the drug. No carcinoids were seen in these rats. An increased incidence of treatment-related ECL cell hyperplasia was observed at the end of 1 year (94% treated vs. 10% controls). By the second year the difference between treated and control rats was much smaller (46% vs. 26%) but still showed more hyperplasia in the treated group. Mutagenesis: Esomeprazole was negative in the Ames mutation test, in the in vivo rat bone marrow cell chromosome aberration test, and the in vivo mouse micronucleus test. Esomeprazole, however, was positive in the in vitro human lymphocyte chromosome aberration test. Impairment of Fertility: The potential effects of esomeprazole on fertility and reproductive performance were assessed using omeprazole studies. Omeprazole at oral doses up to 138 mg/kg/day in rats (about 34 times the human dose of 40 mg/day on a body surface area basis) was found to have no effect on reproductive performance of parental animals. Reproduction Studies: Reproduction studies have been performed in rats at oral doses up to 280 mg/kg/day (about 68 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at oral doses up to 86 mg/kg/day (about 42 times an oral human dose of 40 mg on a body surface area basis) and have revealed no evidence of impaired fertility or harm to the fetus due to esomeprazole.
Levosulpiride
The values expressed as LD50 acute toxicity after oral administration in mice, rats and rabbits were 2450 mg / kg, 2600 mg / kg and greater than 1500 mg / kg. Subacute toxicity tests were conducted by administering the active ingredient in rat, rabbit and dog, daily, for 12-13 weeks. The appearance of any toxic symptoms was not observed at doses of: 25 mg / kg s.c. and 300 mg / kg p.o. in the rat; 250 mg / kg p.o. and 12.5 mg / kg i.m. in rabbits; and 50 and 100 mg / kg p.o. in the dog. To evidentiate the chronic toxicity after administration of the drug for 180-190 days, the following doses were well tolerated: 100 mg / kg p.o. and 20 mg / kg s.c. in the rat; 10 mg / kg i.m. in rabbits; and 20 mg / kg p.o. in the dog. Studies performed in rats and mice, administering the medicine at a dose higher than that expected for man, have shown that levosulpiride do not possess carcinogenic properties. Studies carried out in rats and rabbits have shown that the medicine is not teratogenic. In vitro tests have ruled out that levosulpiride possesses mutagenic properties.
7.0 Description
Each capsule of Zosa L contains 40 mg of esomeprazole (in a gastro-resistant form) and 75 mg of levosulpiride (in a prolonged release form) for oral administration in adults.
Esomeprazole Magnesium
Esomeprazole magnesium is the magnesium salt of esomeprazole, the S-isomer of omeprazole, with gastric proton pump inhibitor activity. Esomeprazole magnesium salt is off-white to pale cream colored powder. It is slightly soluble in water.
Molecular Weight: 713.12 g/mol.
Molecular Formula: C34H36MgN6O6S2.
Chemical Name: 5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl] sulfinyl]benzimidazole, magnesium salt (2:1).
Levosulpiride
Levosulpiride is the active levorotatory enantiomer of the racemic drug sulpiride, a substituted benzamide. Levosulpiride is a dopaminergic antagonist with prokinetic, antiemetic, antidepressant, and antipsychotic properties. Molecular Weight: 341.4 g/mol.
Molecular Formula: C15H23N3O4S.
Chemical Name: N-[[(2S)-1-ethylpyrrolidin-2-yl]methyl]-2-methoxy-5-
sulfamoylbenzamide.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf Life
Refer on pack
8.3 Packaging Information
1 Blister strip of 15 capsules
8.4 Storage and handing Instructions
Store protected from light & moisture at a temperature not exceeding 30°.
Keep out of reach of children.
9.0 Patient counselling information
- Instruct patients to take Zosa L Capsules exactly as prescribed by your doctor. Do not change the dose or stop therapy without consulting to your doctor.
- Instruct patients to take Zosa L capsules on empty stomach, preferably in the morning or at least 1 hour prior to meal. The capsules should be swallowed whole with water and not to be opened, chewed or crushed.
- If you miss a dose, take it as soon as possible. If it is almost time for your next dose, do not take the missed dose. Take the next dose at your regular time. Do not take 2 doses/capsules at the same time to make up for the missed dose.
- Instruct patients not to use this medicine during pregnancy.
- Advise nursing mothers to avoid use of this medicine during lactation or not to breastfeed their infants while on drug therapy.
- This medicine is not recommended for use in children.
- Instruct patients not to share this medication with other people even though symptoms are similar. It may harm them.
- Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Zosa L Capsules and certain other medicines can interact with each other causing serious side effects.
12.0 Date of Revision
28.08.2024
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
- What Zosa-L is and what it is used for
- What you need to know before you take Zosa-L
- How to take Zosa-L
- Possible side effects
- How to store Zosa-L
- Contents of the pack and other information
1. What Zosa-L is and what it is used for
The active ingredients of Zosa-L capsules are Esomeprazole and Levosulpiride.
Esomeprazole belongs to a group of medicines called Proton Pump Inhibitors (PPIs). It acts by reducing the amount of acid made by the stomach.
Levosulpiride belongs to the class of antipsychotic medicines. However, it is primarily used for its gut motility properties i.e., to improve food movement and treats disorders of the stomach and intestines. This medicine works by inhibiting the action of certain specific neurotransmitters in the brain and specific regions of the stomach and the intestine.
Zosa-L capsules are used to treat:
- Gastro-esophageal reflux disease (GERD). GERD is commonly referred to as inflammation of the gullet caused by acid and associated with heartburn. Heartburn is a burning feeling rising from the stomach or lower chest up towards the neck.
2. What you need to know before you take Zosa-L
Do not take Zosa-L if you:
- Patients with known hypersensitivity to esomeprazole or to any substituted benzimidazole derivative or to levosulpiride or to any component of the formulation.
- have Prolactin-releasing pituitary tumour (prolactinoma).
- have hepatic and renal impairment, epilepsy, manic states, hyperprolactinaemia, porphyria, mammary dysplasia, malignant mastopathies, pheochromocytoma.
- have known existing prolongation of cardiac conduction intervals, particularly QTc, patients with significant electrolyte disturbances or underlying cardiac diseases such as congestive heart failure.
- gastro-intestinal haemorrhage, mechanical obstruction or perforation.
Warnings and precautions
Talk to your doctor or pharmacist before taking Zosa-L if you:
- have gastric malignancy such as ulcers, gastrointestinal infections, or gastritis
- have Clostridium Difficile-Associated Diarrhea (CDAD)
- are at risk for osteoporosis-related fractures
- are due to have a specific blood test (Chromogranin A).
Zosa-L may hide the symptoms of other diseases. Therefore, if any of the following happen to you before you start taking Zosa-L or while you are taking it, talk to your doctor straight away:
- You lose a lot of weight for no reason and have problems swallowing.
- You get stomach pain or indigestion.
- You begin to vomit food or blood.
- You pass black stools (blood-stained faeces).
If you have been prescribed Zosa-L “on demand” you should contact your doctor if your symptoms continue or change in character.
Taking a proton pump inhibitor like Zosa-L, especially over a period of more than one year, may slightly increase your risk of fracture in the hip, wrist or spine. Tell your doctor if you have osteoporosis or if you are taking corticosteroids (which can increase the risk of osteoporosis).
Rash and skin symptoms
If you get a rash on your skin, especially in areas exposed to the sun tell your doctor as soon as you can, as you may need to stop your treatment with Zosa-L. Remember to also mention any other ill-effects like pain in your joints.
Serious skin rashes have occurred in patients taking Zosa-L. The rash can involve ulcers of the mouth, throat, nose, genitals and conjunctivitis (red and swollen eyes). These serious skin rashes often come after flu-like symptoms such as fever, headache, body ache. The rash may cover large parts of the body with blistering and peeling of the skin. If at any time during the treatment (even after several weeks) you develop a rash or any of these skin symptoms, stop taking this medicine and contact your doctor immediately.
Children under the age of 12 years
Zosa-L is not recommended for children less than 12 years old.
Other medicines and Zosa-L
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
This is because Zosa-L can affect the way some medicines
work and some medicines can have an effect on Zosa-L. Do not take Zosa-L if you are taking a medicine containing Nelfinavir (used to treat HIV infection). Tell your doctor or pharmacist if you are taking any of the following medicines:
- Atazanavir (used to treat HIV infection).
- Saquinavir
- lopidogrel (used to prevent blood clots).
- Ketoconazole, itraconazole or voriconazole (used to treat infections caused by a fungus).
- Erlotinib (used to treat cancer).
- Citalopram, imipramine or clomipramine (used to treat depression).
- Diazepam (used to treat anxiety, relax muscles or in epilepsy).
- Phenytoin (used in epilepsy).
- Medicines that are used to thin your blood, such as warfarin.
- Cilostazol (used to treat intermittent claudication – a pain in your legs when you walk which is caused by an insufficient blood supply).
- Cisapride (used for indigestion and heartburn).
- Digoxin (used for heart problems).
- Methotrexate (a chemotherapy medicine used in high doses to treat cancer)
- Tacrolimus (organ transplantation).
- Rifampicin (used for treatment of tuberculosis).
- St. John’s wort (Hypericum perforatum) (used to treat depression).
If your doctor has prescribed the antibiotics amoxicillin and clarithromycin as well as Zosa-L to treat ulcers caused by Helicobacter pylori infection, it is very important that you tell your doctor about any other medicines you are taking.
Zosa-L with food and drink
You can take the capsules with food or on an empty stomach.
Pregnancy, breast-feeding and fertility If you are pregnant, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Your doctor will decide whether you can take Zosa-L during this time.
Levosulpiride may be distributed into breast milk. Therefore, you should not take Zosa-L if you are breast-feeding.
Driving and using machines Zosa-L may have an influence on the ability to drive and use machines.
under treatment may experience numbness, dizziness or involuntary movements (dyskinesia); therefore, they should be advised to avoid driving or operating machines
3. How to take Zosa-L
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
- If you are taking this medicine for a long time, your doctor will want to monitor you (particularly if you are taking it for more than a year).
- If your doctor has told you to take this medicine as and when you need it, tell your doctor if your symptoms change.
How much to take
- Your doctor will tell you how many tablets to take and how long to take them for. This will depend on your condition, how old you are and how well your liver works.
The recommended dose is: Once capsule once daily in adults
Use in children
Zosa-L capsules are not recommended for children below 12 years of age.
Elderly
Dose adjustment is not required in the elderly.
If you take more Zosa-L than you should
If you take more Zosa-L than prescribed by your doctor, talk to your doctor or pharmacist straight away.
If you forget to take Zosa-L
- If you forget to take a dose, take it as soon as you remember it. However, if it is almost time for your next dose, skip the missed dose.
- Do not take a double dose (two doses at the same time) to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you notice any of the following serious side effects, stop taking Zosa-L and contact a doctor immediately:
- Adverse drug reaction reports show that Proton Pump Inhibitors like Pantoprazole, Omeprazole, Lansoprazole, Esomeprazole, Rabeprazole etc are associated with acute kidney injury
- The most common adverse events were drowsiness/sedation and endocrine effects.
Other side effects include:
Common (may affect up to 1 in 10 people)
- Headache.
- Effects on your stomach or gut: diarrhoea, stomach pain, constipation, wind (flatulence).
- Feeling sick (nausea) or being sick (vomiting).
- Benign polyps in the stomach.
Uncommon (may affect up to 1 in 100 people)
- Swelling of the feet and ankles.
- Disturbed sleep (insomnia).
- Dizziness, tingling feelings such as “pins and needles”, feeling sleepy.
- Spinning feeling (vertigo).
- Dry mouth.
- Changes in blood tests that check how the liver is working.
- Skin rash, lumpy rash (hives) and itchy skin.
- Fracture of the hip, wrist or spine (if Esomeprazole is used in high doses and over long duration).
Rare (may affect up to 1 in 1,000 people)
- Blood problems such as a reduced number of white cells or platelets. This can cause weakness, bruising or make infections more likely.
- Allergic reactions e.g. fever, angioedema and shock
- Low levels of sodium in the blood. This may cause weakness, being sick (vomiting) and cramps.
- Feeling agitated, confused or depressed.
- Taste changes.
- Eyesight problems such as blurred vision.
- Suddenly feeling wheezy or short of breath (bronchospasm).
- An inflammation of the inside of the mouth.
- An infection called “thrush” which can affect the gut and is caused by a fungus.
- Liver problems, including jaundice which can cause yellow skin, dark urine, and tiredness.
- Hair loss (alopecia).
- Skin rash on exposure to sunshine.
- Joint pains (arthralgia) or muscle pains (myalgia).
- Generally feeling unwell and lacking energy.
- Increased sweating.
Very rare (may affect up to 1 in 10,000 people)
- Changes in blood count including agranulocytosis (lack of white blood cells).
- Aggression.
- Seeing, feeling or hearing things that are not there (hallucinations).
- Severe liver problems leading to liver failure and inflammation of the brain.
- Sudden onset of a severe rash or blistering or peeling skin. This may be associated with a high fever and joint pains (Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms).
- Muscle weakness.
- Severe kidney problems.
- Enlarged breasts in men.
Not known (frequency cannot be estimated from the available data)
- If you are on Zosa-L for more than three months it is possible that the levels of magnesium in your blood may fall. Low levels of magnesium can be seen as fatigue, involuntary muscle contractions, disorientation, convulsions, dizziness or increased heart rate. If you get any of these symptoms, please tell your doctor promptly. Low levels of magnesium can also lead to a reduction in potassium or calcium levels in the blood. Your doctor may decide to perform regular blood tests to monitor your levels of magnesium.
- Inflammation in the gut (leading to diarrhoea).
- Rash, possibly with pain in the joints.
Levosulpiride
Major & minor side effects of Levosulpiride
- Drowsiness
- Breast tenderness
- Irregular menstrual periods
- Gynecomastia
- Constipation
- Abdominal pain and cramps
- Weight gain
- Sleeplessness
- Unusual tiredness and weakness
- Increased salivation
- Decrease in libido
- Fever
- Excessive sweating Change in heart rate
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Drug Safety Reporting” located on the top end of the home page. Website link: https://www.zuventus.com/drug-safety-reporting By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store Zosa-L
- Keep out of reach of children.
- Do not use this medicine after the expiry date which is stated on the blister and the carton after [Exp]. The expiry date refers to the last day of that month.
- Store protected from light & moisture at a temperature not exceeding 30°C.
- Do not throw away medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
Each hard gelatin capsule contains:
Esomeprazole Magnesium Trihydrate IP
equivalent to Esomeprazole…………………………………. 40 mg
(As Gastro-resistant tablet)
Colour: Ferric Oxide (Red) USP-NF
Levosulpiride ……………………………………………..75 mg
(As Prolonged-release uncoated tablet)
Approved colours used in hard gelatin capsule shells.
Presentation/pack size
10 Blister strips of 15 capsules each