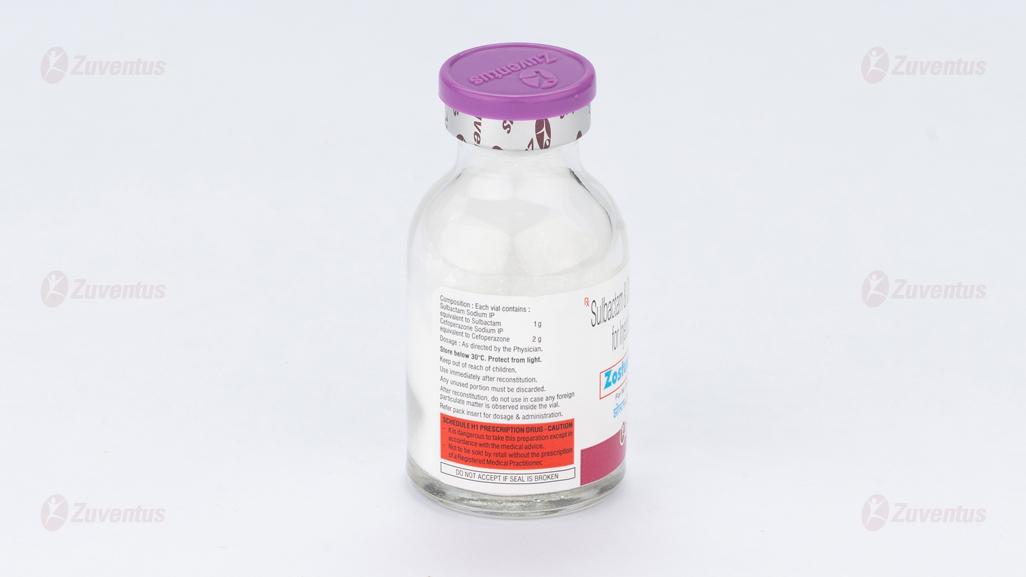
Zostum 3.0 gm Injection
Therapy Area
Anti Infective
1.0 Name of the medicinal product
Sulbactam & Cefoperazone for Injection 500 mg/ 1 g/ 1.5 g/ 2 g/ 3 g
2.0 Qualitative and quantitative composition
Zostum-500
Each vial contains:
Sulbactam Sodium IP equivalent to Sulbactam 250 mg
Cefoperazone Sodium IP equivalent to Cefoperazone 250 mg
This pack contains Sterile Water for Injections IP 5 ml.
Zostum-1g
Each vial contains:
Sulbactam Sodium IP equivalent to Sulbactam 0.5 g
Cefoperazone Sodium IP equivalent to Cefoperazone 0.5 g
This pack contains Sterile Water for Injections IP 20 ml.
Zostum-2g
Each vial contains:
Sulbactam Sodium IP equivalent to Sulbactam 1 g
Cefoperazone Sodium IP equivalent to Cefoperazone 1 g
This pack contains Sterile Water for Injections IP 20 ml.
Zostum-1.5g
Each vial contains:
Sulbactam Sodium IP equivalent to Sulbactam 0.5 g
Cefoperazone Sodium IP equivalent to Cefoperazone 1 g
This pack contains Sterile Water for Injections IP 20 ml.
Zostum-3g
Each vial contains:
Sulbactam Sodium IP equivalent to Sulbactam 1 g
Cefoperazone Sodium IP equivalent to Cefoperazone 2 g
This pack contains Sterile Water for Injections IP 20 ml.
3.0 Dosage form and strength
Vials for IV/IM use
500 mg/ 1 g/ 1.5 g/ 2 g/ 3 g
4.0 Clinical particulars
4.1 Therapeutic indication
The combination of Cefoperazone and Sulbactam is indicated for the treatment of the following infections when caused by susceptible organisms:
- Respiratory tract infections
- Urinary tract infections (upper & lower)
- Endometritis
- Intra-abdominal infections, bone & joint infections
- Septicaemia
- Other infections of the genital tract
- Meningitis
- For a specific subset of patients immunocompromised febrile neutropenic cancer patients
- Skin & soft tissue infections
Combination therapy
Because of the broad spectrum of activity of Cefoperazone and Sulbactam, most infections can be treated adequately with this antibiotic alone. However, it may be used concomitantly with other antibiotics if such combinations are indicated. If an aminoglycoside is used, renal function should be monitored during therapy.
4.2 Posology and method of administration
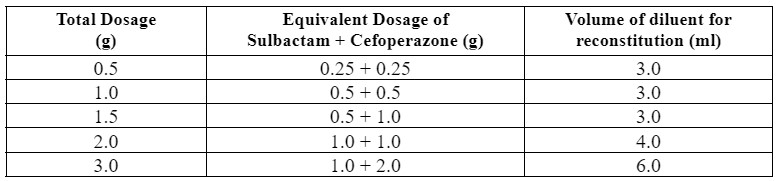
Cefoperazone-Sulbactam is available in 0.5 g, 1.0 g, 1.5 g, 2.0 g, and 3.0 g strength vials.
The usual adult dose of Cefoperazone-Sulbactam is 2 to 4 g per day (i.e. 1 to 2 g per day Cefoperazone activity) given intravenously or intramuscularly in equally divided doses every 12 hours.
In severe or refractory infections, the daily dosage may be increased up to 8 g Cefoperazone-Sulbactam (i.e. 4 g Cefoperazone activity) given intravenously. Patients receiving the 1:1 ratio may require additional cefoperazone administrated separately. The dose should be administered every 12 hours in equally divided doses. The recommended maximum daily dosage of Sulbactam is 4 g (8 g of Cefoperazone-Sulbactam). Dosage regimens of Cefoperazone-Sulbactam should be adjusted in patients with a marked decrease in renal function (creatinine clearance of less than 30 ml/min) to compensate for the reduced clearance of Sulbactam. Patients with creatinine clearance between 15 and 30 ml/min should receive a maximum of 1 g of Sulbactam administered every 12 hours (maximum daily dosage of 2 g Sulbactam), while patients with creatinine clearance of less than 15 ml/min should receive a maximum 500 mg of Sulbactam every 12 hours (maximum daily dosage of 1 g Sulbactam). In severe infections, it may be necessary to administer additional Cefoperazone separately.
The usual dosage of Cefoperazone-Sulbactam in children is 40 to 80 mg/kg/day (i.e. 20 to 40 mg/kg/day of Cefoperazone activity) in 2 to 4 equally divided doses. In serious or refractory infections, these dosages may be increased up to 160 mg/kg/day of Cefoperazone-Sulbactam (i.e. 80 mg/kg/day Cefoperazone activity) in 2 to 4 equally divided doses. For neonates in the first week of life, the drug should be given every 12 hours.
The maximum daily dosage of Sulbactam in pediatrics should not exceed 80 mg/kg/day (160 mg/kg/day cefoperazone-Sulbactam). In cases where a dose above 80 mg/kg/day of cefoperazone is necessary, additional Cefoperazone should be administered separately.
Intravenous Administration
For intermittent infusion, each vial of Cefoperazone-Sulbactam should be reconstituted with the appropriate amount (shown in table) of 5% dextrose in Water, 0.9% Sodium Chloride injection or Sterile Water for injections and then diluted to 5 ml for 500 mg and 20 ml for 1 g, 1.5 g, 2 g, and 3 g with the same solution followed by the administration over 15 to 60 minutes. For intravenous injection, each vial should be reconstituted as above and administered over a minimum of 3 minutes.
Intramuscular Administration
Sterile Water for Injections should be used for reconstitution. For a concentration of Cefoperazone of 250 mg/ml or larger, a two-step dilution is required using Sterile Water followed by 2% lidocaine to approximate a 0.5% lidocaine solution (see below).
4.3 Contraindications
The combination of Cefoperazone and Sulbactam is contraindicated in patients with known allergy to penicillins or any of the cephalosporins.
4.4 Special warnings and precautions for use
Warnings:
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving beta-lactam therapy. These reactions are more apt to occur in individuals with a history of hypersensitivity reactions to multiple allergens. If an allergic reaction occurs, the drug should be discontinued and the appropriate therapy instituted. Serious anaphylactic reaction requires immediate emergency treatment with epinephrine, oxygen, intravenous steroids, and airway management, including intubation treatment.
Precautions:
General
Those precautions that pertain to Sulbactam and Cefoperazone will also pertain to the combination as discussed below.
Cefoperazone is extensively excreted in bile. The serum half-life of Cefoperazone is usually prolonged and urinary excretion of the drug increased in patients with hepatic diseases and/or biliary obstruction. Even with severe hepatic dysfunction, therapeutic concentrations of Cefoperazone are obtained in bile and only a 2 to 4-fold increase in half-life is seen. Dose modification may be necessary in cases of severe biliary obstruction, severe hepatic disease or in cases of renal dysfunction coexistent with either of those conditions. In patients with hepatic dysfunction and concomitant renal impairment, Cefoperazone serum concentration should be monitored and dosage adjusted as necessary. In these cases, dosage should not exceed 2 g/day of Cefoperazone without close monitoring of serum concentrations.
The serum half-life of Cefoperazone is reduced slightly during hemodialysis. Thus, dosing should be scheduled to follow a dialysis period.
As with other antibiotics, Vitamin K deficiency has occurred in a few patients treated with Cefoperazone. The mechanism is most probably related to the suppression of gut flora which normally synthesize this vitamin. Those at risk include patients with poor diet, malabsorption states (e.g., cystic fibrosis), and patients on prolonged intravenous alimentation regimens. Prothrombin time should be monitored in these patients and exogenous vitamin K administered as indicated.
A reaction characterized by flushing, sweating, headache and tachycardia has been reported when alcohol was ingested during and late as the fifth day after Cefoperazone administration. A similar reaction has been reported with certain other cephalosporins and patients should be cautioned concerning ingestion of alcoholic beverages in conjunction with administration of Cefoperazone-Sulbactam. For patients requiring artificial feeding orally or parenterally, solutions containing ethanol should be avoided. As with other antibiotics, overgrowth of non-susceptible organisms may occur during prolonged use of this combination. Patients should be observed carefully during treatment. As with any potent systemic agent, it is advisable to check periodically for organ system dysfunction during extended therapy; this includes renal, hepatic, and hematopoietic systems. This is particularly important in neonates, especially when premature, and other infants.
4.5 Drug Interactions
Combination Therapy
Because of the broad spectrum of activity of sulbactam/cefoperazone, many infections can be treated. However, sulbactam/cefoperazone may be used together with other antibiotics. If an aminoglycoside is used, renal function should be monitored during the course of therapy.
Alcohol
A reaction characterized by flushing, sweating, headache, and tachycardia has been reported when alcohol was ingested during and as late as the fifth day after cefoperazone administration. A similar reaction has been reported with certain other cephalosporins and patients should be cautioned concerning ingestion of alcoholic beverages in conjunction with administration of sulbactam/cefoperazone. For patients requiring artificial feeding orally or parenterally, solutions containing ethanol should be avoided.
Drug Laboratory Test Interactions
A false-positive reaction for glucose in the urine occurs with Benedict's or Fehling's solution.
4.6 Use in special populations
Pregnancy:
Reproduction studies have been performed in rats at doses up to 10 times the human dose and have revealed no evidence of impaired fertility and no teratological findings. Sulbactam and cefoperazone cross the placental barrier. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, sulbactam/cefoperazone should be used during pregnancy only if clearly needed.
Lactation:
Only small quantities of sulbactam and cefoperazone are excreted in human milk. Although both drugs pass poorly into the breast milk of nursing mothers, caution should be exercised when sulbactam/cefoperazone is administered to a nursing mother
Pediatrics population:
Cefoperazone had adverse effects on the testes of prepubertal rats at all doses tested. Subcutaneous administration of 1000 mg/kg/day (approximately 16 times the average adult human dose) resulted in reduced testicular weight, arrested spermatogenesis, reduced germinal cell population, and vacuolation of Sertoli cell cytoplasm. The severity of lesions was dose-dependent in the 100 to 1000 mg/kg/day range; the low dose caused a minor decrease in spermatocytes. This effect has not been observed in adult rats. However, these studies did not evaluate the subsequent development of reproductive function in the rats. The relationship of these findings to humans is unknown.
When Cefoperazone-Sulbactam was given SC to neonatal rats for 1 month, reduced testicular weight and immature tubules were seen in groups given 300+300 mg/kg/day. Because there is a great individual variation in the degree of testicular maturation in rat pups and because immature testicles were found in controls any relation to the study drug is uncertain. No such findings were seen in infant dogs at doses over 10 times the average adult dose.
Usage in Infancy
Cefoperazone-sulbactam has been effectively used in infants. It has not been extensively studied in premature infants or neonates. Therefore, in treating premature infants and neonates potential benefits and possible risks involved should be considered before instituting therapy. Cefoperazone does not displace bilirubin from plasma protein binding sites.
4.7 Effects on the ability to drive and use machines
Clinical experience with sulbactam/cefoperazone indicates that it is unlikely to impair a patient’s ability to drive or use machinery.
4.8 Undesirable effects
Sulbactam/cefoperazone is generally well tolerated. The majority of adverse events are of mild or moderate severity and are tolerated with continued treatment.
The following undesirable effects have been observed and reported during treatment with sulbactam/cefoperazone with the following frequencies: Very common (≥1/10); common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data).
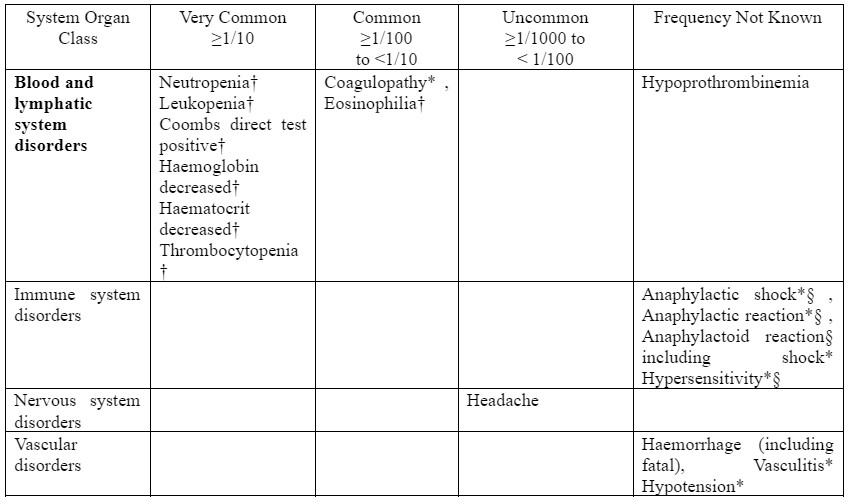
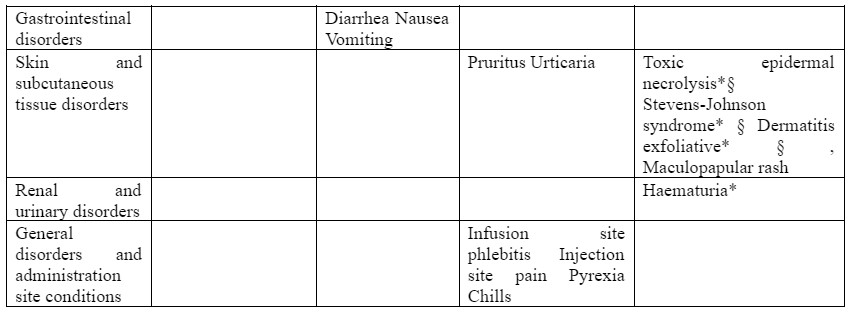
*ADR identified post-marketing
† : In the calculation for laboratory abnormality ADR frequencies, all available laboratory values, including those of subjects with baseline abnormalities, were included. This conservative approach was taken because the raw data did not allow distinction between the subset of subjects with baseline abnormalities who had treatment-emergent significant laboratory changes from those subjects with baseline abnormalities who did not have treatment-emergent significant laboratory changes. For leucocytes, neutrophils, platelets, haemoglobin and haematocrit, only abnormalities are reported in studies. Increases and decreases are not differentiated. § : Fatalities have been reported.
Reporting of side effects
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Limited information exists regarding the acute toxicity of Cefoperazone sodium and sulbactam sodium in humans. Overdosing on the medication would likely lead to symptoms that are mainly extensions of the adverse reactions reported with the drug. It's noteworthy to consider that elevated concentrations of beta-lactam antibiotics in the cerebrospinal fluid (CSF) may result in neurological effects, such as seizures. Additionally, as both Cefoperazone and sulbactam are eliminated from the bloodstream through hemodialysis, these procedures might aid in expediting the removal of the drug from the body in cases of overdose, particularly in patients with impaired renal function.
5.0 Pharmacological properties
5.1 Mechanism of Action
The antibacterial component of sulbactam/cefoperazone is cefoperazone, a third-generation cephalosporin, which acts against sensitive organisms during the stage of active multiplication by inhibiting the biosynthesis of cell wall mucopeptide. Sulbactam does not possess any useful antibacterial activity, except against Neisseriaceae and Acinetobacter. However, biochemical studies with cell-free bacterial synthesis have shown it to be an irreversible inhibitor of most important beta-lactamases produced by beta-lactam antibiotic-resistant organisms.
The potential for sulbactam to prevent the destruction of penicillins and cephalosporins by resistant organisms was confirmed in whole-organism studies using resistant strains in which sulbactam exhibited marked synergy with penicillins and cephalosporins. As sulbactam also binds with some penicillin-binding proteins, sensitive strains are also often rendered more susceptible to sulbactam/Cefoperazone than to Cefoperazone alone.
The combination of Sulbactam and Cefoperazone is active against all organisms sensitive to Cefoperazone. In addition, it demonstrates synergistic activity (up to fourfold reduction in minimum inhibitory concentrations for the combination versus those for each component) in a variety of organisms, most markedly in the following:
Haemophilus influenzae Morganella morganii
Proteus mirabilis Acinetobacter calcoaceticus
Bacteroides species Citrobacter freundii
Klebsiella pneumoniae Enterobacter aerogenes
Staphylococcus species Enterobacter cloacae
Escherichia coli Citrobacter diversus
The combination of Cefoperazone and Sulbactam is active in vitro against a wide variety of clinically significant organisms.
Gram-Positive Organisms
Staphylococcus aureus, penicillinase and non-penicillinase-producing strains
Staphylococcus epidermidis
Streptococcus pneumoniae (formerly Diplococcus pneumoniae)
Streptococcus pyogenes (Group A beta-hemolytic streptococci)
Streptococcus agalactae (Group B beta-hemolytic streptococci)
Most other strains of beta-hemolytic streptococci
Many strains of Streptococcus faecalis (enterococcus)
Gram-Negative Organisms
Escherichia coli Proteus mirabilis
Providencia spp Neisseria gonorrhoeae
Klebsiella spp Proteus vulgaris
Serratia spp (including S. marcescens) Neisseria meningitidis
Enterobacter spp Morganella morganii
Salmonella spp Bordetella pertussis
Shigella spp Yersinia enterocolitica
Haemophilus influenzae Providencia rettgeri
Pseudomonas spp
Anaerobic Organisms
Gram-negative bacilli (including Bacteroides fragilis, other Bacteroides spp, and Fusobacterium spp)
Gram-positive and gram-negative cocci (including Peptococcus, Peptostreptococcus and Veillonella spp)
Gram-positive bacilli (including Clostridium, Eubacterium and Lactobacillus spp)
The following susceptibility ranges have been established for sulbactam/cefoperazone:
For MIC determinations, serial dilutions of sulbactam/cefoperazone in a 1:1 or 1:2 sulbactam/cefoperazone ratio may be used with a broth or agar dilution method. Use of a susceptibility test disc containing 30 mcg of sulbactam and 75 mcg of cefoperazone is recommended. A report from the laboratory of "susceptible" indicates that the infecting organism is likely to respond to sulbactam/cefoperazone therapy, and a report of "Resistant" indicates that the organism is not likely to respond. A report of "Intermediate" suggests that the organism would be susceptible to sulbactam/cefoperazone if a higher dosage is used or if the infection is confined to tissues or fluids where high antibiotic levels are attained.
The following quality control limits are recommended for 30 mcg/75 mcg sulbactam/cefoperazone susceptibility discs:
| Control Strain | Zone Size (mm) |
| Acinetobacter spp., ATCC 43498 | 26-32 |
| Pseudomonas aeuriginosa, ATCC 27853 | 22-28 |
| Escherichia coli, ATCC 25922 | 27-33 |
| Staphylococcus aureus, ATCC 25923 | 23-30 |
5.2 Pharmacodynamic properties
Pharmacotherapeutic Class: Antibacterial for systemic use.
ATC Code: J01DA.
Zostum® is a combination of sulbactam sodium/cefoperazone sodium. Sulbactam sodium is a derivative of the basic penicillin nucleus. It is an irreversible beta-lactamase inhibitor for parenteral use only. Cefoperazone sodium is a third-generation semisynthetic broad-spectrum cephalosporin antibiotic for parenteral use only.
5.3 Pharmacokinetic properties
Distribution
Mean peak sulbactam and cefoperazone concentrations after the administration of 2 g (1:1 ratio) of sulbactam/cefoperazone (1 g sulbactam + 1 g of cefoperazone) intravenously over 5 minutes to healthy volunteers were 130 and 236.8 mcg/ml respectively following a single dose. This reflects the larger volume of distribution for sulbactam (Vd = 18.0-27.6 L) compared to cefoperazone (Vd = 10.2-11.3 L).
Mean peak sulbactam and cefoperazone concentrations after the administration of 4.5 grams (1:2 ratio) of sulbactam/cefoperazone (1.5 g sulbactam + 3 g cefoperazone) intravenously over 15 minutes to healthy volunteers were 88.3 mcg/ml and 416.1 mcg/ml, respectively following a single dose.
Peak serum concentrations of sulbactam and cefoperazone following a dose of 1.5 g of cefoperazone sulbactam (0.5 g sulbactam + 1 g cefoperazone) administered by intramuscular route to healthy volunteers were respectively 11.0 mcg/ml and 45.3 mcg/ml following the first dose and were 29.9 mcg/ml and 58.4 mcg/ml respectively after the 7th dose administered every 12 hours.
Elimination
Approximately 84% of the sulbactam dose and 25% of the cefoperazone dose administered with sulbactam/cefoperazone is excreted by the kidney. Most of the remaining dose of cefoperazone is excreted in the bile. After sulbactam/cefoperazone administration the mean half-life for sulbactam is about 1 hour while that for cefoperazone is 1.7 hours. Serum concentrations have been shown to be proportional to the dose administered. These values are consistent with previously published values for the agents when given alone.
After intramuscular administration of 1.5 g sulbactam/cefoperazone (0.5 g sulbactam, 1 g cefoperazone) peak serum concentrations of sulbactam and cefoperazone are seen from 15 minutes to 2 hours after administration. Mean peak serum concentrations were 19.0 and 64.2 mcg/ml for sulbactam and cefoperazone, respectively.
After multiple dosing no significant changes in the pharmacokinetics of either component of sulbactam/cefoperazone have been reported and no accumulation has been observed when administered every 8 to 12 hours.
Use in Renal Dysfunction
In patients with different degrees of renal function who were administered sulbactam/cefoperazone, the total body clearance of sulbactam was highly correlated with estimated creatinine clearance. Patients who are functionally anephric showed a significantly longer half-life of sulbactam (mean 6.9 and 9.7 hours in separate studies).
Haemodialysis significantly altered the half-life, total body clearance, and volume of distribution of sulbactam. No significant differences have been observed in the pharmacokinetics of cefoperazone in renal failure patients.
Use in Elderly
The pharmacokinetics of sulbactam/cefoperazone have been studied in elderly individuals with renal insufficiency and compromised hepatic function. Both sulbactam and cefoperazone exhibited longer half-life, lower clearance, and larger volumes of distribution when compared to data from normal volunteers. The pharmacokinetics of sulbactam correlated well with the degree of renal dysfunction while for cefoperazone there was a good correlation with the degree of hepatic dysfunction.
Pediatric Population
Studies conducted in pediatrics have shown no significant changes in the pharmacokinetics of the components of sulbactam/cefoperazone compared to adult values. The mean half-life in children has ranged from 0.91 to 1.42 hours for sulbactam and from 1.44 to 1.88 hours for cefoperazone.
Both sulbactam and cefoperazone distribute well in a variety of tissues and fluids including bile, gall bladder, skin, appendix, fallopian tubes, ovary, uterus and others. There is no evidence of any pharmacokinetic drug interaction between sulbactam and cefoperazone when administered together in the form of Zostum.
Cefoperazone does not displace bilirubin from plasma protein binding sites.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Pharmaco-toxicology studies either with single or repeated administrations on various animal species have shown that Sulbactam /Cefoperazone is well tolerated.
7.0 Description
Cefoperazone is a third-generation cephalosporin antimicrobial agent. It has increased activity against Gram-negative organisms. Chemically Cefoperazone sodium is sodium salt of (6R,7R)-7-[(2R)-2-{[(4-ethyl-2,3-dioxopiperazin-1-yl)carbonyl]amino}-2-(4-hydroxyphenyl)acetamido]-3-{[(1-methyl-1H-1,2,3,4-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid.
Sulbactam is a beta lactamase inhibitor. It is a derivative of the basic penicillin nucleus. Chemically Sulbactam sodium is sodium penicillinate sulfone and is an off-white crystalline powder highly soluble in water.
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Incompatibilities
Aminoglycosides
Solutions of sulbactam/cefoperazone and aminoglycosides should not be directly mixed, since there is a physical incompatibility between them. If combination therapy with sulbactam/cefoperazone and an aminoglycoside is contemplated this can be accomplished by sequential intermittent intravenous infusion provided that separate secondary intravenous tubing is used and that the primary intravenous tubing is adequately irrigated with an approved diluent between doses. It is also suggested that doses of sulbactam/cefoperazone be administered throughout the day at times as far removed from administration of the aminoglycoside as possible.
Lactated Ringer’s Solution
Initial reconstitution with Lactated Ringer's Solution should be avoided since this mixture is incompatible. However, a two-step dilution process involving initial reconstitution in water for injection will result in a compatible mixture when further diluted with Lactated Ringer's Solution at a Sulbactam concentration of 5 mg/ml.
Lidocaine
Initial reconstitution with 2% lidocaine HCl solution should be avoided since this mixture is incompatible. However, a two-step dilution process involving initial reconstitution in water for injection will result in a compatible mixture when further diluted with 2% lidocaine HCl solution. Similarly, after appropriate initial reconstitution with water for injection, Cefoperazone-Sulbactam could be further diluted with 2% lidocaine hydrochloride to yield solutions containing up to 250 mg Cefoperazone and 125 mg Sulbactam per ml in 0.5% lidocaine HCI solution.
8.2 Shelf life
Refer on the pack.
8.3 Packaging Information
Presentation:
Zostum-500: A vial of 500 mg with SWFI IP 5 ml.
Zostum-1g: A vial of 1 g with SWFI IP 20 ml.
Zostum-1.5g: A vial of 1.5 g with SWFI IP 20 ml.
Zostum-2g: A vial of 2 g with SWFI IP 20 ml.
Zostum-3g: A vial of 3 g with SWFI IP 20 ml.
8.4 Storage and handling instructions
Store below 30°C. Protect from light. Keep out of reach of children. After reconstitution, do not use it in case any foreign particulate matter is observed inside the vial.
9.0 Patient counselling information
Patients should be counseled that antibacterial drugs including Zostum® should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold).
When Zostum® is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed.
Skipping doses or not completing the full course of therapy may:
1. decrease the effectiveness of the immediate treatment and
2. increase the likelihood that bacteria will develop resistance and will not be treated by Zostum® or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibacterial drugs which usually ends when the drug is discontinued.
Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drug. If this occurs, patients should contact their physician as soon as possible.
About leaflet
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What ZOSTUM® injection is and what it is used for
2. What you need to know before you are given ZOSTUM® injection
3. How ZOSTUM® injection is given
4. Possible side effects
5. How to store ZOSTUM® injection
6. Contents of the pack and other information
1. What ZOSTUM® injection is and what it is used for
ZOSTUM® is a combination antibiotic comprising two distinct medications: Cefoperazone and sulbactam. This medication is utilized in the treatment of a diverse range of bacterial infections. It works by killing bacteria that cause infections. Cefoperazone belongs to the class of third-generation cephalosporin antibiotics, while sulbactam functions as a beta-lactamase inhibitor. The synergy between these two components enhances the efficacy of the treatment by preventing the degradation of cefoperazone by beta-lactamase enzymes produced by certain bacteria. This mechanism enables the antibiotic to exert its effects over a prolonged period, making it particularly effective against multidrug-resistant bacteria or in situations necessitating broad-spectrum coverage. This combination is often used in the treatment of infections caused by multidrug-resistant bacteria or in situations where broad-spectrum coverage is required.
ZOSTUM® injection is used for the treatment of:
- Urinary tract infection (infection of the kidney, bladder, ureter urethra, etc.),
- Respiratory tract infections (infection of the lung, pneumonia, etc.)
- Septicaemia (blood infection)
- Meningitis (brain infection)
- Genital tract infection (infection of the vagina, uterus, etc.)
- the abdomen and abdominal wall (peritonitis).
- Skin & soft tissue infection
- Bone & joint infection.
2. What you need to know before you are given ZOSTUM® injection
You must not be given ZOSTUM® injection if:
- You are allergic to ZOSTUM®
- You have had a sudden or severe allergic reaction to penicillin or similar antibiotics (such as cephalosporins, carbapenems, or monobactams). The signs include sudden swelling of the throat or face which might make it difficult to breathe or swallow, sudden swelling of the hands, feet, and ankles, and a severe rash that develops quickly.
- After reconstitution, do not use in case any foreign particulate matter is observed inside the vial.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you are given ZOSTUM® injection if:
- You experience serious and occasionally fatal hypersensitivity (anaphylactic) reactions. These reactions are more apt to occur in individuals with a history of hypersensitivity reactions to multiple allergens.
- You have biliary obstruction, liver or kidney problems.
- You have other illnesses, such as haemolytic anaemia (a reduction in your red blood cells that may make your skin pale yellow and cause weakness or breathlessness).
If you need a blood or urine test
ZOSTUM® can affect the results of urine tests for sugar and give false positive results. If you are having tests:
- Tell the person taking the sample that you have been given ZOSTUM®.
ZOSTUM® in babies:
- It has not been extensively studied in premature infants or neonates. Thus, your doctor will decide if ZOSTUM® can be given to such babies, based on the potential benefits and possible risks involved before instituting therapy.
Other medicines and ZOSTUM®
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
In particular, tell your doctor or pharmacist if you are taking:
- Alcohol
Pregnancy and breast-feeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. The doctor will consider the benefit of treating you with ZOSTUM® against the risk to your baby.
Driving and using machines
Clinical experience with ZOSTUM® indicates that it is unlikely to impair a patient's ability to drive or use machinery.
3. How ZOSTUM® injection is given
ZOSTUM® injection is usually given by a doctor or nurse. It can be given as a drip (intravenous infusion) or as an injection directly into a vein or into a muscle. ZOSTUM® injection is made up by the doctor, pharmacist, or nurse.
The usual dose
Your doctor will decide the correct dose of ZOSTUM® for you. The dose will depend on the severity and type of infection; whether you are on any other antibiotics; your weight and age; and how well your kidneys and liver are working. The number of days or weeks that you are given ZOSTUM® depends on what sort of infection you have.
Adults
The usual adult dose ranges from 2 to 4 g in equally divided doses twice a day depending on the severity and type of infection. If you have a severe infection, your doctor will give you a higher dose (up to 8 g in equally divided doses twice a day). If your daily dose is higher than 2 g, you may receive it as a single dose once a day or as two separate doses.
Children aged > 7 days
The usual dosage of ZOSTUM® in children is 40 to 80 mg/kg/day (i.e. 20 to 40 mg/kg/day of Cefoperazone activity) in 2 to 4 equally divided doses, depending on the severity and type of infection. If you have a severe infection, your doctor will give you a higher dose up to 160 mg/kg/day of ZOSTUM® in 2 to 4 equally divided doses.
Newborn babies (0-7 days)
For neonates in the first week of life, ZOSTUM® will be given every 12 hours. The maximum daily dosage of Sulbactam in these patients should not exceed 80 mg/kg/day (160 mg/kg/day ZOSTUM®). In cases where a dose above 80 mg/kg/day of Cefoperazone is necessary, additional Cefoperazone will be administered separately.
People with liver and kidney problems
You may be given a different dose than the usual dose. Your doctor will decide how much ZOSTUM® you will need and will check you closely depending on the severity of the liver and kidney disease.
If you are given more ZOSTUM® than you should
If you accidentally receive more than your prescribed dose, contact your doctor or nearest hospital straight away.
If you forget to use the ZOSTUM® injection
If you miss an injection, you should have it as soon as possible. However, if it is almost time for your next injection, skip the missed injection. Do not take a double dose (two injections at the same time) to make up for a missed dose.
If you stop using ZOSTUM® injection
Do not stop taking ZOSTUM® unless your doctor tells you to. If you have any further questions on the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may happen with this medicine:
Gastrointestinal: They are frequently observed and diarrhea/loose stools have been reported most frequently followed by nausea and vomiting. They are seen in 3.6 to 10.8% of the patients.
Dermatologic Reactions: As with all penicillins and cephalosporins, hypersensitivity presenting as rash, itching, and fever are seen in 0.8 to 1.3% of the patients, and are more likely to occur in patients with a history of allergies, particularly to penicillin.
Haematology: On long-term use, there is a slight decrease in neutrophil count. Some patients develop a positive direct Coombs test.
Decreased haemoglobin or haematocrit can occur. A brief period of increased eosinophil and decreased platelet count with a decline in serum prothrombin levels is observed.
Miscellaneous adverse events (headache, fever, injection pain, chills) is seen in less than 1% of patients.
Local Reactions: Occasionally, transient pain may occur on intramuscular injection. As with other cephalosporins and penicillins, ZOSTUM® injected in veins may lead to inflammation of the vein at the infusion site.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effects with the help of your treating physician.
5. How to store ZOSTUM® injection
Do not use ZOSTUM® injection after the expiry date which is printed on the label and carton.
Do not store above 25°C. Protect from light.
Keep out of the sight and reach of children.
Your doctor, pharmacist, or nurse will know how to store ZOSTUM® Injection properly.
6. Contents of the pack and other information
What ZOSTUM® injection contains
Zostum-500: A vial of 500 mg (Sulbactam / Cefoperazone 250 mg each) with SWFI IP 5 ml.
Zostum-1g: A vial of 1 g (Sulbactam / Cefoperazone 500 mg each) with SWFI IP 20 ml.
Zostum-1.5g: A vial of 1.5 g (Sulbactam 500 mg & Cefoperazone 1 g) with SWFI IP 20 ml.
Zostum-2g: A vial of 2 g (Sulbactam / Cefoperazone 1 g each) with SWFI IP 20 ml.
Zostum-3g: A vial of 3 g (Sulbactam 1 g / Cefoperazone 2 g) with SWFI IP 20 ml.
12.0 Date of revision
This leaflet was last revised in May 2024.