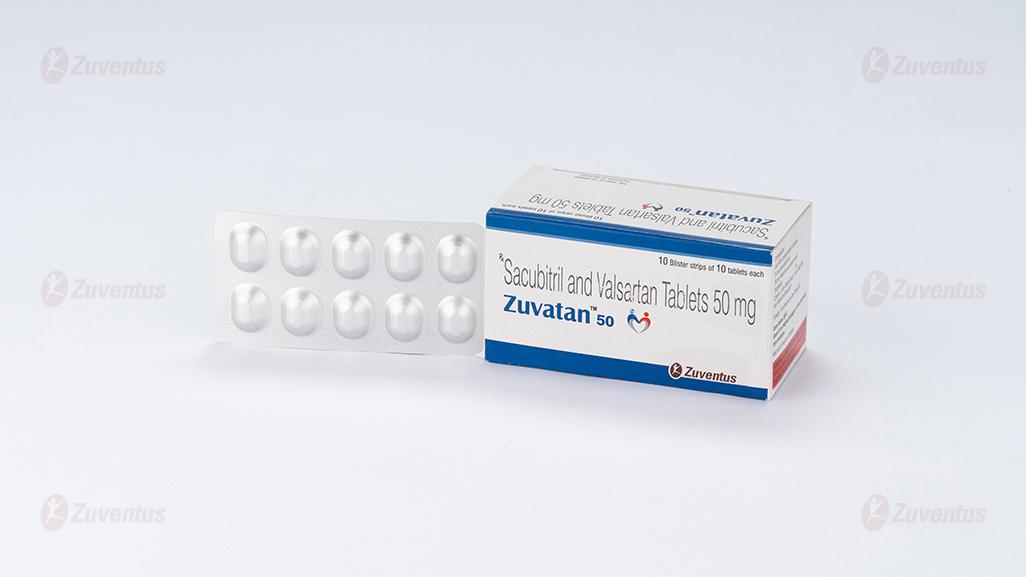
Zuvatan 50 Tablets
Therapy Area
Cardiology
1.0 Generic name
Sacubitril and Valsartan Tablets 50 mg / 100 mg / 200 mg
2.0 Qualitative and quantitative composition
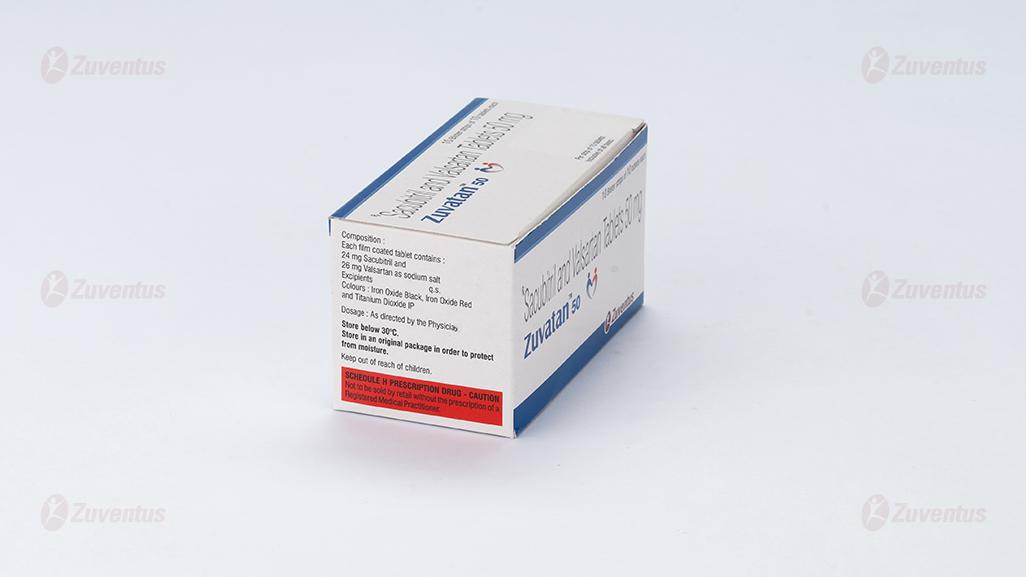
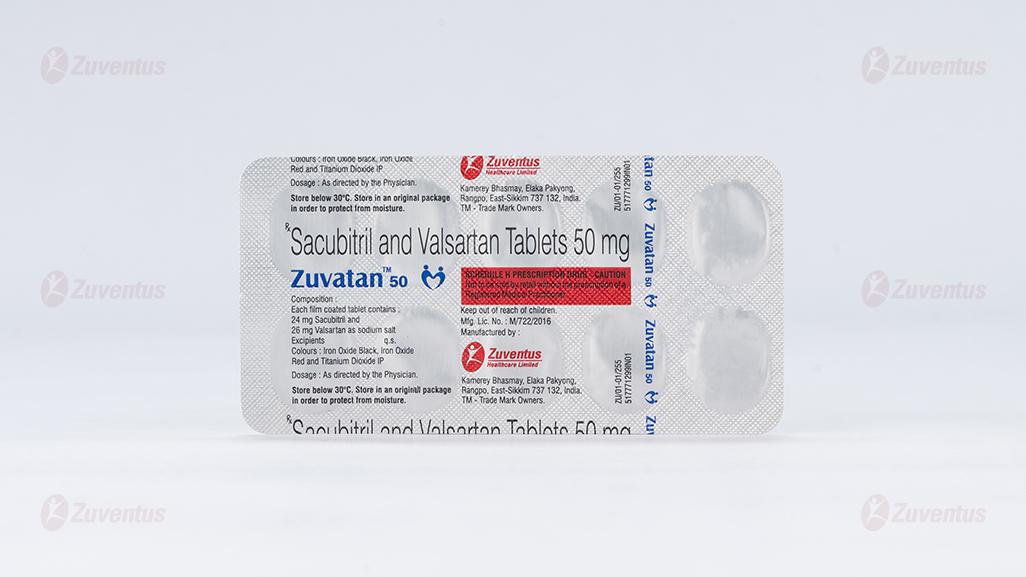
Zuvatan 50
Each film coated tablet contains :
24 mg Sacubitril and 26 mg Valsartan as sodium salt
Excipients q.s.
Colours : Iron Oxide Black, Iron Oxide Red and Titanium Dioxide IP
Zuvatan 100
Each film coated tablet contains :
49 mg Sacubitril and 51 mg Valsartan as sodium salt
Excipients q.s
Colours : Iron Oxide Yellow, Iron Oxide Red and Titanium Dioxide IP
Zuvatan 200
Each film coated tablet contains :
97 mg Sacubitril and 103 mg Valsartan as sodium salt
Excipients q.s.
Colours : Iron Oxide Black, Iron Oxide Red and Titanium Dioxide IP
3.0 Dosage form and strength
Tablet, 50 (24 + 26) mg, 100 (49 + 51) mg, 200 (97 + 103) mg
4.0 Clinical particulars
4.1 Therapeutic indication
To reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.
4.2 Posology and method of administration
General considerations
Zuvatan is contraindicated with concomitant use of an angiotensin-converting enzyme (ACE) inhibitor. If switching from an ACE inhibitor to Zuvatan allow a washout period of 36 hours between administration of the two drugs
Adult heart failure
The recommended starting dose of Zuvatan is 100 mg orally twice-daily. Double the dose of Zuvatan after 2 to 4 weeks to the target maintenance dose of 200 mg twice daily, as tolerated by the patient.
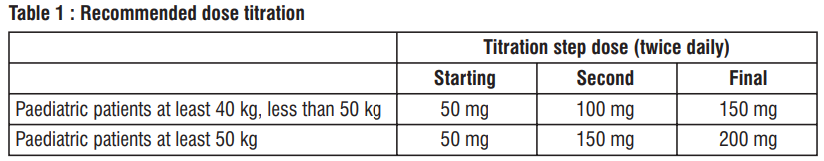
Dose adjustment for patients not taking an ace inhibitor or arb or previously taking low doses of these agents
In patients not currently taking an ACE inhibitor or an angiotensin II receptor blocker (ARB) and for patients previously taking low doses of these agents, start Zuvatan at half the usually recommended starting dose. After initiation, increase the dose every 2 to 4 weeks in adults and every 2 weeks in paediatric patients to follow the recommended dose escalation thereafter.
Dose adjustment for severe renal impairment
In adults and paediatric patients with severe renal impairment (eGFR < 30 mL/min/1.73 m ), start Zuvatan at half the usually recommended starting dose. After initiation, increase the dose to follow the recommended dose escalation thereafter.
No starting dose adjustment is needed for mild or moderate renal impairment.
Dose adjustment for hepatic impairment
In adults and paediatric patients with moderate hepatic impairment (Child-Pugh B classication), start Zuvatan at half the usually recommended starting dose. After initiation, increase the dose to follow the recommended dose escalation thereafter.
No starting dose adjustment is needed for mild hepatic impairment. Use in patients with severe hepatic impairment is not recommended.
Method of administration
Oral use
Zuvatan may be administered with or without food. The tablets must be swallowed with a glass of water.
4.3 Contraindications
- Hypersensitivity to the active substances or to any of the excipients listed in the formulation.
- Concomitant use with ACE inhibitors. Zuvatan must not be administered until 36 hours after discontinuing ACE inhibitor therapy.
- Known history of angioedema related to previous ACE inhibitor or ARB therapy.
- Hereditary or idiopathic angioedema.
- Concomitant use with Aliskiren-containing medicinal products in patients with diabetes mellitus or in patients with renal impairment (eGFR <60 ml/min/1.73 m2 ).
- Severe hepatic impairment, biliary cirrhosis and cholestasis.
- Second and third trimesters of pregnancy.
4.4 Warning and precautions
Dual blockade of the renin-angiotensin-aldosterone system (RAAS)
The combination of Sacubitril / Valsartan with an ACE inhibitor is contraindicated due to the increased risk of angioedema. Sacubitril / Valsartan must not be initiated until 36 hours after taking the last dose of ACE inhibitor therapy. If treatment with Sacubitril / Valsartan is stopped, ACE inhibitor therapy must not be initiated until 36 hours after the last dose of Sacubitril / Valsartan. The combination of Sacubitril / Valsartan with direct renin inhibitors such as Aliskiren is not recommended. The combination of Sacubitril / Valsartan with Aliskiren-containing medicinal products is contraindicated in patients with diabetes mellitus or 2 in patients with renal impairment (eGFR <60 ml/min/1.73 m ). Zuvatan contains valsartan, and therefore should not be co-administered with another ARB containing medicinal product.
Hypotension
Treatment should not be initiated unless SBP is ≥ 100 mmHg. Patients with SBP < 100 mmHg were not studied. Cases of symptomatic hypotension have been reported in patients treated with Sacubitril / Valsartan during clinical studies, especially in patients ≥ 65 years old, patients with renal disease and patients with low SBP (< 112 mmHg). When initiating therapy or during dose titration with Sacubitril / Valsartan, blood pressure should be monitored routinely. If hypotension occurs, temporary down-titration or discontinuation of Sacubitril / Valsartan is recommended. Dose adjustment of diuretics, concomitant antihypertensive and treatment of other causes of hypotension (e.g. hypovolaemia) should be considered. Symptomatic hypotension is more likely to occur if the patient has been volume-depleted, e.g. by diuretic therapy, dietary salt restriction, diarrhoea or vomiting. Sodium and/or volume depletion should be corrected before starting treatment with Sacubitril / Valsartan, however, such corrective action must be carefully weighed against the risk of volume overload.
Impaired renal function
Evaluation of patients with heart failure should always include assessment of renal function. Patients with mild and moderate renal impairment are more at risk of developing hypotension). There is very limited clinical experience 2 in patients with severe renal impairment (estimated GFR < 30 ml/min/1.73 m ) and these patients may be at greatest risk of hypotension. There is no experience in patients with end-stage renal disease and use of Sacubitril / Valsartan is not recommended.
Worsening renal function
Use of Sacubitril / Valsartan may be associated with decreased renal function. The risk may be further increased by dehydration or concomitant use of non-steroidal anti-inammatory agents (NSAIDs). Down-titration should be considered in patients who develop a clinically signicant decrease in renal function.
Hyperkalaemia
Treatment should not be initiated if the serum Potassium level is > 5.4 mmol/l. Use of Sacubitril / Valsartan may be associated with an increased risk of hyperkalaemia, although hypokalaemia may also occur. Monitoring of serum Potassium is recommended, especially in patients who have risk factors such as renal impairment, diabetes mellitus or hypoaldosteronism or who are on a high Potassium diet or on mineralocorticoid antagonists. If patients experience clinically signicant hyperkalaemia adjustment of concomitant medicinal products, or temporary down–titration or discontinuation is recommended. If serum Potassium level is > 5.4 mmol/l discontinuation should be considered.
Angioedema
Angioedema has been reported in patients treated with Sacubitril / Valsartan. If angioedema occurs, Sacubitril / Valsartan should be immediately discontinued and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms has occurred. It must not be re-administered. In cases of confirmed angioedema where swelling has been conned to the face and lips, the condition has generally resolved without treatment, although antihistamines have been useful in relieving symptoms. Angioedema associated with laryngeal oedema may be fatal. Where there is involvement of the tongue, glottis or larynx likely to cause airway obstruction, appropriate therapy, e.g. Adrenaline solution 1 mg / 1 ml (0.3 - 0.5 ml), and/or measures necessary to ensure a patent airway, should be promptly administered. Patients with a prior history of angioedema were not studied. As they may be at higher risk for angioedema, caution is recommended if Sacubitril / Valsartan is used in these patients. Sacubitril / Valsartan is contraindicated in patients with a known history of angioedema related to previous ACE inhibitor or ARB therapy or with hereditary or idiopathic angioedema. Black patients have an increased susceptibility to develop angioedema.
Patients with renal artery stenosis
Sacubitril / Valsartan may increase blood urea and serum creatinine levels in patients with bilateral or unilateral renal artery stenosis. Caution is required in patients with renal artery stenosis and monitoring of renal function is recommended.
Patients with NYHA functional classification IV
Caution should be exercised when initiating Sacubitril / Valsartan in patients with NYHA functional classication IV due to limited clinical experience in this population.
B-type natriuretic peptide (BNP)
BNP is not a suitable biomarker of heart failure in patients treated with Sacubitril / Valsartan because it is a Neprilysin substrate.
Patients with hepatic impairment
There is limited clinical experience in patients with moderate hepatic impairment (Child-Pugh B classication) or with AST/ALT values more than twice the upper limit of the normal range. In these patients, exposure may be increased and safety is not established. Caution is therefore recommended when using it in these patients. Sacubitril / Valsartan is contraindicated in patients with severe hepatic impairment, biliary cirrhosis or cholestasis (Child-Pugh C classification).
Psychiatric disorders
Psychiatric events such as hallucinations, paranoia and sleep disorders, in context of psychotic events, have been associated with Sacubitril / Valsartan use. If a patient experiences such events, discontinuation of Sacubitril / Valsartan treatment should be considered
4.5 Drug interactions
Interactions resulting in a contraindication
ACE inhibitors
The concomitant use of Sacubitril / Valsartan with ACE inhibitors is contraindicated, as the concomitant inhibition of Neprilysin (NEP) and ACE may increase the risk of angioedema. Sacubitril / Valsartan must not be started until 36 hours after taking the last dose of ACE inhibitor therapy. ACE inhibitor therapy must not be started until 36 hours after the last dose of Sacubitril / Valsartan.
Aliskiren
The concomitant use of Sacubitril / Valsartan with Aliskiren-containing medicinal products is contraindicated in patients with diabetes mellitus or 2 in patients with renal impairment (eGFR < 60 ml/min/1.73 m ). The combination of Sacubitril / Valsartan with direct renin inhibitors such as Aliskiren is not recommended. Combination of Sacubitril / Valsartan with Aliskiren is potentially associated with a higher frequency of adverse events such as hypotension, hyperkalaemia and decreased renal function (including acute renal failure).
Interactions resulting in concomitant use not being recommended
Sacubitril / Valsartan contains valsartan, and therefore should not be co-administered with another ARB containing medicinal product.
Interactions requiring precautions
OATP1B1 and OATP1B3 substrates, e.g. statins In vitro data indicate that Sacubitril inhibits OATP1B1 and OATP1B3 transporters. Zuvatan may therefore increase the systemic exposure of OATP1B1 and OATP1B3 substrates such as statins. Co-administration of Sacubitril / Valsartan increased the Cmax of atorvastatin and its metabolites by up to 2-fold and AUC by up to 1.3-fold. Caution should be exercised when co-administering Sacubitril / Valsartan with statins. No clinically relevant interaction was observed when simvastatin and Zuvatan were co-administered.
PDE5 inhibitors including Sildenafil
Addition of a single dose of Sildenafil to Sacubitril / Valsartan at steady state in patients with hypertension was associated with a significantly greater blood pressure reduction compared to administration of Sacubitril / Valsartan alone. Therefore, caution should be exercised when Sildenafil or another PDE5 inhibitor is initiated in patients treated with Sacubitril / Valsartan.
Potassium
Concomitant use of Potassium-sparing diuretics (Triamterene, Amiloride), mineralocorticoid antagonists (e.g. Spironolactone, Eplerenone), Potassium supplements, salt substitutes containing Potassium or other agents (such as Heparin) may lead to increases in serum Potassium, and to increases in serum creatinine. Monitoring of serum Potassium is recommended if Sacubitril / Valsartan is co-administered with these agents.
Non-steroidal anti-inflammator y agents (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors
In elderly patients, volume-depleted patients (including those on diuretic therapy), or patients with compromised renal function, concomitant use of Sacubitril / Valsartan and NSAIDs may lead to an increased risk of worsening of renal function. Therefore, monitoring of renal function is recommended when initiating or modifying treatment in patients on Sacubitril / Valsartan who are taking NSAIDs concomitantly.
Lithium
Reversible increases in serum Lithium concentrations and toxicity have been reported during concomitant administration of Lithium with ACE inhibitors or angiotensin II receptor antagonists including Sacubitril / Valsartan. Therefore, this combination is not recommended. If the combination proves necessary, careful monitoring of serum Lithium levels is recommended. If a diuretic is also used, the risk of Lithium toxicity may be increased further.
Furosemide
Co-administration of Sacubitril / Valsartan and Furosemide had no effect on the pharmacokinetics of Sacubitril / Valsartan but reduced Cmax and AUC of Furosemide by 50% and 28%, respectively. While there was no relevant change in urine volume, the urinary excretion of sodium was reduced within 4 hours and 24 hours after coadministration. The average daily dose of Furosemide was unchanged from baseline until the end of the PARADIGM-HF study in patients treated with Sacubitril / Valsartan.
Nitrates, e.g. Nitroglycerine
There was no drug-drug interaction between Sacubitril / Valsartan and intravenously administered Nitroglycerin with regard to blood pressure reduction. Co-administration of Nitroglycerin and Sacubitril / Valsartan was associated with a treatment difference of 5 bpm in heart rate compared to the administration of Nitroglycerin alone. A similar effect on the heart rate may occur when Sacubitril / Valsartan is co-administered with sublingual, oral or transdermal nitrates. In general no dose adjustment is required.
OATP and MRP2 transporters
The active metabolite of Sacubitril (LBQ657) and valsartan are OATP1B1, OATP1B3, OAT1 and OAT3 substrates; Valsartan is also a MRP2 substrate. Therefore, co-administration of Sacubitril / Valsartan with inhibitors of OATP1B1, OATP1B3, OAT3 (e.g. Rifampicin, Ciclosporin), OAT1 (e.g. Tenofovir, Cidofovir) or MRP2 (e.g. Ritonavir) may increase the systemic exposure of LBQ657 or Valsartan. Appropriate care should be exercised when initiating or ending concomitant treatment with such medicinal products.
Metformin
Co-administration of Sacubitril / Valsartan with metformin reduced both Cmax and AUC of Metformin by 23%. The clinical relevance of these findings is unknown. Therefore, when initiating therapy with Sacubitril / Valsartan in patients receiving metformin, the clinical status of the patient should be evaluated.
No significant interaction
No clinically meaningful drug-drug interaction was observed when Sacubitril / Valsartan was co-administered with Digoxin, Warfarin, Hydrochlorothiazide, Amlodipine, Omeprazole, Carvedilol or a combination of Levonorgestrel/Ethinyl Estradiol.
4.6 Special populations
Pregnancy
The use of Sacubitril / Valsartan is not recommended during the rst trimester of pregnancy and is contraindicated during the second and third trimesters of pregnancy. There are no data from the use of Sacubitril / Valsartan in pregnant women. Animal studies with Sacubitril / Valsartan have shown reproductive toxicity.
Breast-feeding
It is not known whether Sacubitril / Valsartan is excreted in human milk. The components Sacubitril and Valsartan, were excreted in the milk of lactating rats. Because of the potential risk for adverse reactions in breast-fed new-borns/infants, it is not recommended during breast-feeding. A decision should be made whether to abstain from breast-feeding or to discontinue Zuvatan while breast-feeding, taking into account the importance of Sacubitril / Valsartan to the mother.
Fertility
There are no available data on the effect of Sacubitril / Valsartan on human fertility. No impairment of fertility was demonstrated in studies with it in male and female rats.
4.7 Effects on ability to drive and use machines
Sacubitril / Valsartan has a minor influence on the ability to drive and use machines. When driving vehicles or operating machines it should be taken into account that occasionally dizziness or fatigue may occur.
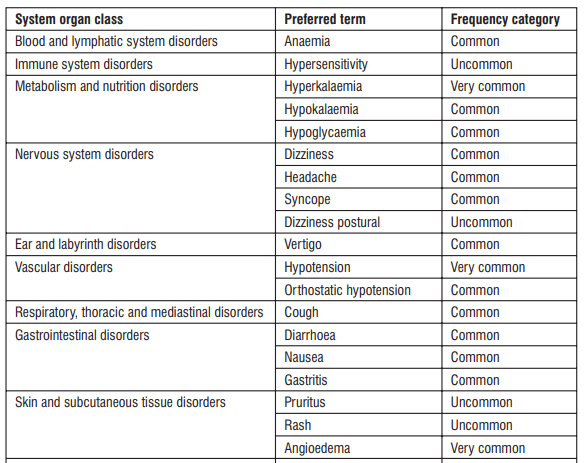
4.8 Undesirable Effects
The most commonly reported adverse reactions during treatment with Sacubitril / Valsartan were hypotension, hyperkalaemia and renal impairment. Angioedema was reported in patients treated with Sacubitril / Valsartan.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit / risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to : medico@zuventus.com
Website : https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Limited data are available with regard to overdose in humans. A single dose of 583 mg Sacubitril / 617 mg Valsartan and multiple doses of 437 mg Sacubitril / 463 mg Valsartan (14 days) were studied in healthy volunteers and were well tolerated. Hypotension is the most likely symptom of overdose due to the blood pressure lowering effects of Sacubitril / Valsartan. Symptomatic treatment should be provided. The medicinal product is unlikely to be removed by haemodialysis due to high protein binding.
5.0 Pharmacological properties
5.1 Mechanism of action
Sacubitril / Valsartan exhibits the mechanism of action of an angiotensin receptor Neprilysin inhibitor by simultaneously inhibiting Neprilysin (neutral endopeptidase; NEP) via LBQ657, the active metabolite of the prodrug Sacubitril, and by blocking the angiotensin II type-1 (AT1) receptor via Valsartan. The complementary cardiovascular benefits of Sacubitril / Valsartan in heart failure patients are attributed to the enhancement of peptides that are degraded by Neprilysin, such as natriuretic peptides (NP), by LBQ657 and the simultaneous inhibition of the effects of angiotensin II by Valsartan. NPs exert their effects by activating membrane-bound guanylyl cyclase-coupled receptors, resulting in increased concentrations of the second messenger cyclic guanosine monophosphate (cGMP), which could result in vasodilation, natriuresis and diuresis, increased glomerular fltration rate and renal blood flow, inhibition of renin and aldosterone release, reduction of sympathetic activity, and anti-hypertrophic and anti-fibrotic effects.
Valsartan inhibits detrimental cardiovascular and renal effects of angiotensin II by selectively blocking the AT1 receptor, and also inhibits angiotensin II-dependent aldosterone release. This prevents sustained activation of the renin-angiotensin-aldosterone system that would result in vasoconstriction, renal sodium and fluid retention, activation of cellular growth and proliferation, and subsequent maladaptive cardiovascular remodelling.
5.2 Pharmacodynamic Properties
The pharmacodynamic effects of Sacubitril / Valsartan were evaluated after single and multiple dose administrations in healthy subjects and in patients with heart failure, and are consistent with simultaneous Neprilysin inhibition and RAAS blockade. In a 7-day valsartan-controlled study in patients with reduced ejection fraction (HFrEF), administration of Sacubitril / Valsartan resulted in an initial increase in natriuresis, increased urine cGMP, and decreased plasma levels of mid-regional pro-atrial natriuretic peptide (MR-proANP) and Nterminal prohormone brain natriuretic peptide (NTproBNP) compared to valsartan. In a 21-day study in HFrEF patients, Sacubitril / Valsartan signicantly increased urine ANP and cGMP and plasma cGMP, and decreased plasma NT-proBNP, aldosterone and endothelin-1 compared to baseline. The AT1-receptor was also blocked as evidenced by increased plasma renin activity and plasma renin concentrations. In the PARADIGM-HF study, Sacubitril / Valsartan decreased plasma NT-proBNP and increased plasma BNP and urine cGMP compared with Enalapril. BNP is not a suitable biomarker of heart failure in patients treated with Sacubitril / Valsartan because BNP is a neprilysin substrate. NT-proBNP is not a Neprilysin substrate and is therefore a more suitable biomarker.
In a thorough QTc clinical study in healthy male subjects, single doses of Sacubitril / Valsartan 194 mg Sacubitril / 206 mg Valsartan and 583 mg Sacubitril / 617 mg Valsartan had no effect on cardiac repolarisation. Neprilysin is one of multiple enzymes involved in the clearance of amyloid- (A ) from the brain and cerebrospinal fluid (CSF). Administration of Sacubitril / Valsartan 194 mg Sacubitril / 206 mg Valsartan once daily for two weeks to healthy subjects was associated with an increase in CSF A 1-38 compared to placebo; there were no changes in concentrations of CSF A 1-40 and 1-42. The clinical relevance of this finding is not known
5.3 Pharmacokinetic properties
Absorption
Following oral administration, Sacubitril / Valsartan dissociates into valsartan and the prodrug Sacubitril. Sacubitril is further metabolised to the active metabolite LBQ657. These reach peak plasma concentrations in 2 hours, 1 hour, and 2 hours, respectively. The oral absolute bioavailability of Sacubitril and Valsartan is estimated to be more than 60% and 23%, respectively.
Following twice daily dosing of Sacubitril / Valsartan, steady-state levels of Sacubitril, LBQ657 and valsartan are reached in three days. At steady state, Sacubitril and Valsartan do not accumulate significantly, while LBQ657 accumulates 1.6-fold. Administration with food has no clinically significant impact on the systemic exposures of Sacubitril, LBQ657 and valsartan. Sacubitril / Valsartan can be administered with or without food
Distribution
Sacubitril, LBQ657 and valsartan are highly bound to plasma proteins (94 - 97%). Based on the comparison of plasma and CSF exposures, LBQ657 crosses the blood brain barrier to a limited extent (0.28%). The average apparent volume of distribution of Valsartan and Sacubitril were 75 litres to 103 litres, respectively.
Biotransformation
Sacubitril is readily converted to LBQ657 by carboxylesterases 1b and 1c; LBQ657 is not further metabolised to a significant extent. Valsartan is minimally metabolised, as only about 20% of the dose is recovered as metabolites. A hydroxyl metabolite of Valsartan has been identified in plasma at low concentrations (< 10%). Since CYP450-enzyme-mediated metabolism of Sacubitril and Valsartan is minimal, co-administration with medicinal products that impact CYP450 enzymes is not expected to impact the pharmacokinetics. In vitro metabolism studies indicate that potential for CYP450 based drug interactions is low since there is limited metabolism of Sacubitril / Valsartan via CYP450 enzymes. Sacubitril / Valsartan does not induce or inhibit CYP450 enzymes.
Elimination
Following oral administration, 52 - 68% of Sacubitril (primarily as LBQ657) and ~13% of Valsartan and its metabolites are excreted in urine; 37 - 48% of Sacubitril (primarily as LBQ657) and 86% of valsar tan and its metabolites are excreted in faeces.
Sacubitril, LBQ657 and Valsartan are eliminated from plasma with a mean elimination half-life (T½) of approximately 1.43 hours, 11.48 hours, and 9.90 hours, respectively.
6.0 Nonclinical properties
6.1 Animal toxicology or pharmacology
Non-clinical data (including studies with Sacubitril and Valsartan components and/or Sacubitril / Valsartan) reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential and fertility.
7.0 Description
Zuvatan (Sacubitril and Valsartan) is a combination of a Neprilysin inhibitor and an angiotensin II receptor blocker.
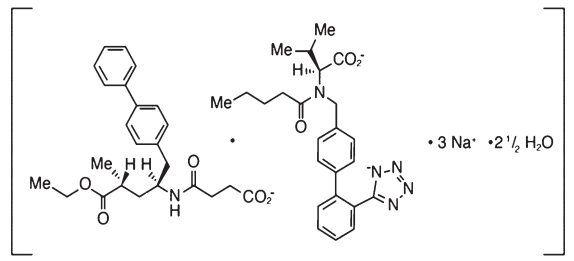
Following oral administration, the complex dissociates into Sacubitril (which is further metabolized to LBQ657) and Valsartan. The complex is chemically described as Octadecasodiumhexakis(4-{[(1S,3R)-1-([1,1´- biphenyl]-4-ylmethyl)-4-ethoxy-3- methyl-4-oxobutyl]amino}-4-oxobutanoate)hexakis(N-pentanoyl-N-{[2´- (1H-tetrazol-1-id-5-yl)[1,1´-biphenyl]-4-yl]methyl}-L-valinate)—water (1/15).
Its empirical formula is C48H55N6O8Na3 2.5 H2O. Its molecular mass is 957.99 g/mol and its schematic structural formula is
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable
8.2 Shelf-life
Refer on the pack.
8.3 Packaging information
Zuvatan 50 : Alu-Alu blister strip of 10 tablets.
Zuvatan 100 : Alu-Alu blister strip of 10 tablets.
Zuvatan 200 : Alu-Alu blister strip of 10 tablets.
8.4 Storage and handing instructions
Store below 30°C. Store in an original package in order to protect from moisture.
Keep out of reach of children.
9.0 Patient counselling information
What is Zuvatan ?
Zuvatan is a prescription medicine used to treat adults with long-lasting (chronic) heart failure to help reduce the risk of death and hospitalization. Zuvatan works better when the heart cannot pump a normal amount of blood to the body.
Pregnancy
Advise female patients of childbearing age about the consequences of exposure to Zuvatan during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physicians as soon as possible
Angioedema
Advise patients to discontinue use of their previous ACE inhibitor or ARB. Advise patients to allow a 36 hour wash-out period if switching from or to an ACE inhibitor.
What is the most important information I should know about Zuvatan ?
Zuvatan can harm or cause death to your unborn baby. Talk to your doctor about other ways to treat heart failure if you plan to become pregnant. If you get pregnant during treatment with Zuvatan, tell your doctor right away.
Do not take Zuvatan if you :
- Are allergic to any of the ingredients in Zuvatan. See the end of this Patient Information leaflet for a complete list of ingredients in Zuvatan.
- Have had an allergic reaction including swelling of your face, lips, tongue, throat, or trouble breathing while taking a type of medicine called an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB).
- Take an ACE inhibitor medicine. Do not take Zuvatan for at least 36 hours before or after you take an ACE inhibitor medicine. Talk with your doctor or pharmacist before taking Zuvatan if you are not sure if you take an ACE inhibitor medicine.
- Have diabetes and take a medicine that contains Aliskiren.
Before taking Zuvatan, tell your doctor about all of your medical conditions, including if you :
- Have a history of hereditary angioedema.
- Have kidney or liver problems.
- Are pregnant or plan to become pregnant.
- Are breastfeeding or plan to breastfeed. It is not known if Zuvatan passes into your breast milk. You and your doctor should decide if you will take Zuvatan or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using Zuvatan with certain other medicines may affect each other. Using Zuvatan with other medicines can cause serious side effects.
Especially tell your doctor if you take :
- Potassium supplements or a salt substitute.
- Nonsteroidal anti-inflammatory drugs (NSAIDs).
- Lithium
- Other medicines for high blood pressure or heart problems such as an ACE inhibitor, ARB, or Aliskiren.
How should I take Zuvatan ?
- Take Zuvatan exactly as your doctor tells you to take it.
- Take Zuvatan 2 times each day. Your doctor may change your dose of Zuvatan during treatment.
- If your child cannot swallow tablets, or if tablets are not available in the prescribed strength, your pharmacist will prepare Zuvatan as a liquid suspension for your child. If your child switches between taking the tablet and the suspension, your doctor will adjust the dose as needed. Shake the bottle of suspension well before measuring the dose of medicine to give to your child.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose,
- Do not take the missed dose. Take the next dose at your regular time.
- If you take too much Zuvatan, call your doctor right away.
12.0 Date of issue
15 February 2023
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
- What Zuvatan is and what it is used for
- What you need to know before you take Zuvatan
- How to take Zuvatan
- Possible side effects
- How to store Zuvatan
- Contents of the pack and other information
1. What Zuvatan is and what it is used for
Zuvatan is a medicine containing an angiotensin receptor neprilysin inhibitor. It delivers two active substances, sacubitril and valsartan.
Zuvatan is used to treat a type of long-term heart failure in adults.
This type of heart failure occurs when the heart is weak and cannot pump enough blood to the lungs and the rest of the body. The most common symptoms of heart failure are breathlessness, fatigue, tiredness and ankle swelling.
2. What you need to know before you take Zuvatan
Do not take Zuvatan
- if you are allergic to sacubitril, valsartan
- if you are taking another type of medicine called an angiotensin converting enzyme (ACE) inhibitor (for example enalapril, lisinopril or ramipril). ACE inhibitors are used to treat high blood pressure or heart failure. If you have been taking an ACE inhibitor, wait for 36 hours’ after taking the last dose before you start to take Zuvatan (see “Other medicines and Zuvatan”).
- if you or a member of your family have ever had a reaction called angioedema (swelling of the face, lips, tongue and/or throat, difficulties in breathing) when taking an ACE inhibitor or an angiotensin receptor blocker (ARB) (such as valsartan, telmisartan or irbesartan).
- if you have diabetes or impaired kidney function and you are being treated with a blood pressure lowering medicine containing aliskiren (see “Other medicines and Zuvatan”).
- if you have severe liver disease
- if you are more than 3 months pregnant (it is also better to avoid this medicine in early pregnancy, see “Pregnancy and breast-feeding”).
If any of the above applies to you, do not take Zuvatan and talk to your doctor.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before or when taking Zuvatan:
- if you are being treated with an angiotensin receptor blocker (ARB) or aliskiren (see “Do not take Zuvatan”).
- if you have ever had angioedema (see “Do not take Zuvatan” and section 4 “Possible side effects”).
- if you have low blood pressure or are taking any other medicines that reduce your blood pressure (for example, a diuretic) or are suffering from vomiting or diarrhoea, especially if you are aged 65 years or more, or if you have kidney disease and low blood pressure.
- if you have severe kidney disease.
- if you are suffering from dehydration.
- if your kidney artery has narrowed.
- if you have liver disease.
- if you experience hallucinations, paranoia or changes in sleeping pattern.
Your doctor may check the amount of potassium in your blood at regular intervals during Zuvatan treatment.
If any of the above applies to you, tell your doctor, pharmacist or nurse before you take Zuvatan.
Children and adolescents
Do not give this medicine to children (aged below 18 years) because it has not been studied in this age group.
Other medicines and Zuvatan
Tell your doctor, pharmacist or nurse if you are taking, have recently taken or might take any other medicines. It may be necessary to change the dose, to take other precautions, or even to stop taking one of the medicines. This is particularly important for the following medicines:
- ACE inhibitors. Do not take Zuvatan with ACE inhibitors. If you have been taking an ACE inhibitor, wait 36 hours after taking the last dose of the ACE inhibitor before starting to take Zuvatan (see “Do not take Zuvatan”).
- If you stop taking Zuvatan, wait 36 hours after taking your last dose of Zuvatan before starting an ACE inhibitor. other medicines used to treat heart failure or lower blood pressure, such as angiotensin receptor blockers or aliskiren (see “Do not take Zuvatan”).
- some medicines known as statins that are used to lower high cholesterol levels (for example atorvastatin).
- sildenafil, a medicine used to treat erectile dysfunction or lung hypertension. medicines that increase the amount of potassium in the blood.
- These include potassium supplements, salt substitutes containing potassium, potassium-sparing medicines and heparin.
- painkillers of the type called non-steroidal anti-inflammatory medicines (NSAIDs) or selective cyclooxygenase-2 (Cox-2) inhibitors.
- If you are taking one of these, your doctor may want to check your kidney function when starting or adjusting treatment (see “Warnings and precautions”). lithium, a medicine used to treat some types of psychiatric illness.
- furosemide, a medicine belonging to the type known as diuretics, which are used to increase the amount of urine you produce.
- nitroglycerine, a medicine used to treat angina pectoris.
- some types of antibiotics (rifamycin group), ciclosporin (used to prevent rejection of transplanted organs) or antivirals such as ritonavir (used to treat HIV/AIDS). metformin, a medicine used to treat diabetes.
If any of the above applies to you, tell your doctor or pharmacist before you take Zuvatan. Pregnancy and breastfeeding
Pregnancy
You must tell your doctor if you think that you are (or might become) pregnant. Your doctor will normally advise you to stop taking this medicine before you become pregnant or as soon as you know you are pregnant, and will advise you to take another medicine instead of Zuvatan.
This medicine is not recommended in early pregnancy, and must not be taken when more than 3 months pregnant, as it may cause serious harm to your baby if it is used after the third month of pregnancy.
Breast-feeding
Zuvatan is not recommended for mothers who are breast-feeding. Tell your doctor if you are breast-feeding or about to start breast-feeding.
Driving and using machines
Before you drive a vehicle, use tools or operate machines, or carry out other activities that require concentration, make sure you know how Zuvatan affects you. If you feel dizzy or very tired while taking this medicine, do not drive a vehicle, cycle or use any tools or machines.
3. How to take Zuvatan
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
You will usually start by taking 24 mg/26 mg or 49 mg/51 mg twice a day (one tablet in the morning and one tablet in the evening). Your doctor will decide your exact starting dose based on which medicines you have been taking previously. Your doctor will then adjust the dose depending on how you respond to the treatment until the best dose for you is found.
The usual recommended target dose is 97 mg/103 mg twice a day (one tablet in the morning and one tablet in the evening).
Patients taking Zuvatan can develop low blood pressure (dizziness, light-headedness), a high level of potassium in the blood (which would be detected when your doctor performed a blood test) or decreased kidney function. If this happens, your doctor may reduce the dose of any other medicine you are taking, temporarily reduce your Zuvatan dose, or stop your Zuvatan treatment completely.
Swallow the tablets with a glass of water. You can take Zuvatan with or without food. Splitting or crushing of the tablets is not recommended.
If you take more Zuvatan than you should
If you have accidentally taken too many Zuvatan tablets, or if someone else has taken your tablets, contact your doctor immediately. If you experience severe dizziness and/or fainting, tell your doctor as
quickly as possible and lie down.
If you forget to take Zuvatan
It is advisable to take your medicine at the same time each day. However, if you forget to take a dose, you should simply take the next one at the scheduled time. Do not take a double dose to make up for a forgotten tablet.
If you stop taking Zuvatan
Stopping your treatment with Zuvatan may cause your condition to get worse. Do not stop taking your medicine unless your doctor tells you to. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects may be serious.
- Stop taking Zuvatan and seek immediate medical attention if you notice any swelling of the face, lips, tongue and/or throat, which may cause difficulties in breathing or swallowing. These may be signs of angioedema (an uncommon side effect which may affect up to 1 in 100 people).
Other possible side effects:
If any of the side effects listed below become severe, tell your doctor or pharmacist.
Very common (may affect more than 1 in 10 people)
- low blood pressure (dizziness, light-headedness)
- high level of potassium in the blood (shown in a blood test)
- decreased renal function (renal impairment)
Common (may affect up to 1 in 10 people)
- cough
- dizziness
- diarrhoea
- low level of red blood cells (shown in a blood test)
- tiredness
- (acute) renal failure (severe kidney disorder)
- low level of potassium in the blood (shown in a blood test)
- headache
- fainting
- weakness
- feeling sick (nausea)
- low blood pressure (dizziness, light-headedness) when switching from sitting or lying to standing position
- gastritis (stomach pain, nausea)
- spinning sensation
- low level of sugar in the blood (shown in a blood test)
Uncommon (may affect up to 1 in 100 people)
- allergic reaction with rash and itching
- dizziness when switching from sitting to standing position
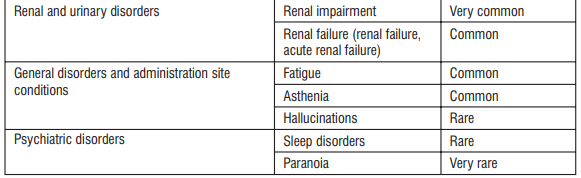
Rare (may affect up to 1 in 1,000 people)
- hallucinations
- changes in sleeping pattern
Very rare (may affect up to 1 in 10,000 people)
- paranoia
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Zuvatan
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of that month.
This medicine does not require any special temperature storage conditions. Store in the original package in order to protect from moisture. Do not use this medicine if you notice that the pack is damaged or shows signs of tampering. Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Zuvatan contains
- The active substances are sacubitril and valsartan.
Zuvatan® 24 mg/26 mg film-coated tablets
Each film-coated tablet contains:
24 mg Sacubitril and 26 mg Valsartan as sodium salt
Excipients. q.s.
Colours:
Iron Oxide Black, Iron Oxide Red and Titanium Dioxide IP
Zuvatan® 49 mg/51 mg film-coated tablets
Each film-coated tablet contains:
49 mg Sacubitril and 51 mg Valsartan as sodium salt Excipients. q.s.
Colours: Iron Oxide Black, Iron Oxide Red and Titanium Dioxide IP Z
uvatan® 97 mg/103 mg film-coated tablets Each film-coated tablet contains: 97 mg
Sacubitril and 103 mg Valsartan as sodium salt Excipients. q.s.
Colours: Iron Oxide Black, Iron Oxide Red and Titanium Dioxide IP
Pack size
Zuvatan® film-coated tablets are available as follows: A blister strip of 10 tablets