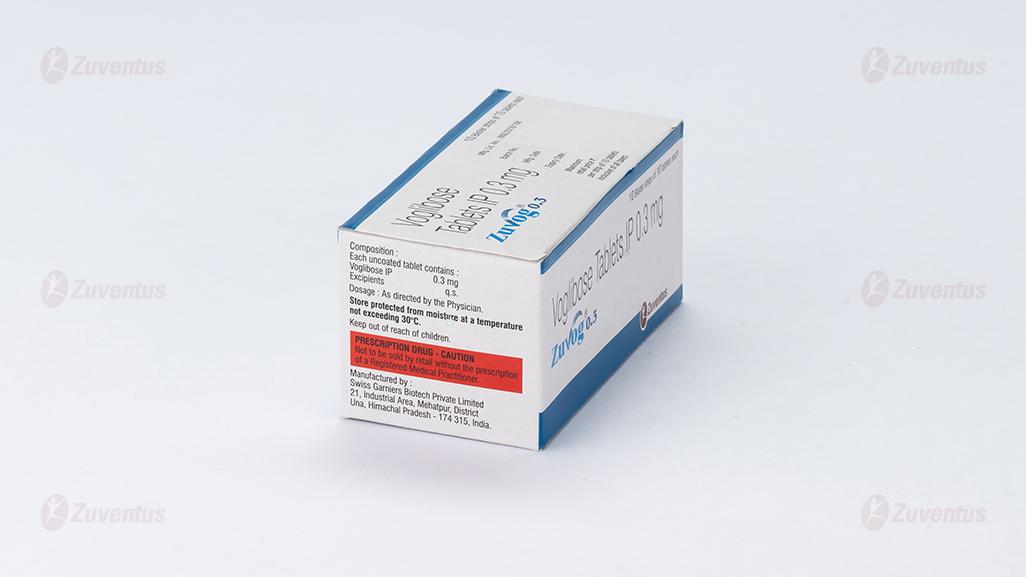
Zuvog 0.3 mg Tablet
Therapy Area
Anti-diabetic
1.0 Generic Name
Voglibose Tablets 0.2mg / 0.3mg
2.0 Qualitative and quantitative composition
Each uncoated tablet contains:
Voglibose……………….0.2mg / 0.3mg
Excipients q.s.
3.0 Dosage form and strength
Dosage Form: Tablets.
Dosage Strength: Voglibose 0.2 mg and 0.3 mg per tablet.
4.0 Clinical particulars
4.1 Therapeutic Indication
For improvement of post prandial hyperglycaemia in diabetic mellitus only when diet and/or exercise or oral hypoglycemic drugs or insulin preparations in addition to diet and /or exercise do not result adequate glycemic control.
4.2 Posology and method of administration
ZUVOG is orally administered in a single dose of 0.2 mg three times daily just before or with each meal.
ZUVOG should be administered in conjunction with diet treatment or diet plus a sulphonylurea.
In case of inadequate effect, the single dose may be increased up to 0.3 mg under close observation of the course of the disease.
4.3 Contraindication
- Hypersensitivity to voglibose or to any component of the formulation.
- Diabetic ketoacidosis, diabetic pre-coma.
- Severe infection, before and after surgery, serious trauma.
- Gastrointestinal obstruction or predisposed to it
4.4 Special warnings and precautions for use
Hypoglycaemia
While on voglibose tablets, patients should be instructed and explained to recognize hypoglycaemic symptoms and its management.
Loss of Control of Blood Glucose
When diabetes patients are exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of control of blood glucose may occur. At such times, temporary insulin therapy may be necessary.
4.5 Drugs interactions
Anti-Diabetic Drugs: When voglibose is used in combination with derivative(s) of sulfonylamide, sulfonylurea or biguanide, or with insulin, hypoglycemic symptoms may occur. Therefore, when used in combination with any of these drugs, care should be taken, such as to initiate therapy with lower dosage.
Drugs Affecting Glycemic Control: When voglibose is administered concomitantly with drugs that enhance or diminish the hypoglycemic action of antidiabetic drugs, caution should be taken as this might additionally delay the action of voglibose on the absorption of carbohydrates. Examples of drugs enhancing the hypoglycemic action of antidiabetic drugs include alpha-blockers, salicylic acid preparations, monoamine oxidase inhibitors, and fibrate derivatives. Examples of drugs diminishing the hypoglycemic action of antidiabetic drugs include epinephrine, adrenocortical hormone, and thyroid hormone.
Warfarin: Voglibose does not affect the pharmacokinetics of warfarin; hence, it can be safely administered along with warfarin.
4.6 Use in special populations
Pregnant Women
Pregnancy Category B. The safety of voglibose in pregnancy has not been established. Animal studies do not indicate harmful effects with respect to pregnancy, embryonal or fetal development, parturition or post-natal development. However, no adequate and well controlled studies have been done in pregnant women. Therefore, voglibose should be given to pregnant women only when the potential benefits to the mother outweigh the possible hazards to the fetus.
Lactating Women
Animal studies (e.g., rats) have revealed a suppressive action of voglibose on body weight increase in newborns probably due to suppression of milk production due to reduced carbohydrate absorption. Although the levels of voglibose reached in human milk are exceedingly low, it is recommended that voglibose may not be administered to lactating women. When the administration of voglibose is unavoidable, nursing should be discontinued.
Paediatric Patients
The safety and effectiveness of voglibose in children has not been established. Thus, Zuvog Tablets are not recommended for use in paediatric patients.
Geriatric
Patients Since elderly patients generally have a physiological hypofunction, it is desirable to initiate therapy with lower dosage. Moreover, this drug should be carefully administered under close observation, throughout the course of the disease, with careful attention to the blood sugar level and the onset of gastrointestinal symptoms.
Renal Impairment Patients
Voglibose is poorly absorbed after oral doses and renal excretion is negligible. Thus, in general, no dosage adjustment is required in patients with renal dysfunction.
Effects on ability to drive and use machines
4.7 Effects on ability to drive and use machines
With voglibose, effect on ability to drive a vehicle or operating machinery has not been reported.
4.8 Undesirable effects
- Gastrointestinal: Gastrointestinal adverse events such as diarrhoea, loose stools, abdominal pain, constipation, anorexia, nausea, vomiting, or heartburn may occur with the use of voglibose. Also, abdominal distention, increased flatus, and intestinal obstruction like symptoms due to an increase in intestinal gas may occur with use of voglibose.
- Hypersensitivity: Rash and pruritus may rarely occur. In such cases, voglibose should be discontinued immediately.
- Hepatic: When voglibose is administered to patients with liver cirrhosis, hyperammonia may worsen with the development of constipation followed by disturbance of consciousness.
- Laboratory Tests: Elevation of SGOT (serum glutamate oxaloacetate), SGPT (serum glutamate pyruvate transaminase), LDH (lactate dehydrogenase), alpha-GPT (alpha-glutamate pyruvate transaminase) or alkaline phosphatase may infrequently occur.
- Hypoglycemia: When voglibose is used in combination with other antidiabetic drugs, hypoglycemia may occur (0.1% to <5%).
- Psychoneurologic: Headache may rarely occur.
- Hematologic: Anemia, thrombocytopenia, and leucopoenia may rarely occur.
- Others: Numbness, edema of face, blurred vision, hot flushes, malaise, weakness, hyperkalemia, increased serum amylase, decreased HDL-cholesterol, diaphoresis, or alopecia may occur rarely with the use of voglibose.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: https://www.zuventus.co.in/drug-safety-reporting
4.9 Overdose
Voglibose is unlikely to produce hypoglycemia in overdose, but abdominal discomfort and diarrhoea may occur. If overdose occurs, supportive and symptomatic treatment should be provided.
5.0 Pharmacological properties
5.1 Mechanism of Action
Voglibose competitively and reversibly inhibits the alpha glucosidase enzymes (e.g., glucoamylase, sucrase, maltase, and isomaltase) in the brush border of the small intestine. Alpha-glucosidase enzymes are essential for hydrolysis/decomposition of complex carbohydrates (starch, dextrin, polysaccharides, and disaccharides) into simpler carbohydrates (such as glucose/dextrose or fructose). Inhibition of these enzymes leads to delay in the absorption of glucose into the bloodstream resulting in improvement of post-prandial hyperglycemia.
5.2 Pharmacodynamic properties
Voglibose exerts its activity in the intestinal tract. Alpha glucosidase enzyme normally converts complex carbohydrates into simple monosaccharides (glucose) which can be absorbed through the intestine.
The action of voglibose depends on an inhibition of intestinal enzymes (alpha-glucosidase) involved in the degradation of ingested disaccharides, oligosaccharides, and polysaccharides into monosaccharides. Inhibition of these enzyme systems reduces/delays the rate of digestion of complex carbohydrates. As there is delay in digestion of complex carbohydrates, monosaccharides release slowly and hence absorbed more slowly into the blood i.e., less glucose absorption from intestine into the blood circulation. Voglibose, thus dose dependently reduces the postprandial rise in blood glucose level.
Although voglibose reduces the impact of complex carbohydrates on blood sugar level, it does not affect/inhibit absorption of glucose from the intestine.
Alpha-glucosidase inhibitors such as voglibose do not stimulate insulin release and therefore do not result in hypoglycemia. Voglibose improves post-prandial hyperglycemia and thereby lowers abnormally high levels of glycosylated hemoglobin. Voglibose is highly useful in elderly patients or in patients with predominantly post-prandial hyperglycemia.
5.3 Pharmacokinetic properties
Absorption: Voglibose is poorly absorbed after oral doses. Plasma concentrations after oral doses have usually been undetectable. Following repeated administration to healthy subjects (n=6) in a single dose of 0.2 mg, 3 times a day, for 7 consecutive days, voglibose was not detected in plasma or urine. Similarly, when voglibose was administered to healthy male adults (n=10) as a single dose of 2 mg, voglibose was not detected in plasma or urine.
Distribution: After ingestion of voglibose, the majority of active unchanged drug remains in the lumen of the gastrointestinal tract to exert its pharmacological activity.
Metabolism: Voglibose is metabolized by intestinal enzymes and by the microbial flora. Excretion: Voglibose is mainly excreted in the feces.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
A study demonstrated that voglibose can potentiate CCl4 (carbon tetrachloride) and APAP (acetaminophen) hepatotoxicity in rats by inducing hepatic CYP2E1.
7.0 Description
Voglibose is an alpha-glucosidase inhibitor used to manage postprandial blood glucose levels in patients with type 2 diabetes mellitus.
Chemical name: (1S, 2S, 3R, 4S, 5S)-5-(1,3-dihydroxypropan-2-ylamino)-1-(hydroxymethyl) cyclohexane-1,2,3,4-tetrol.
Molecular formula: C10H21NO7
Molecular mass: 267.28 g/mol.
Structural formula:

8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on pack
8.3 Packaging information
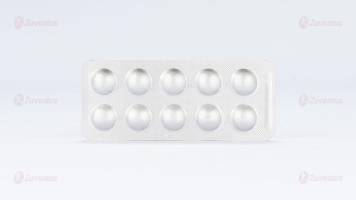
Zuvog 0.2 / 0. 3: A blister strip of 10 tablets
8.4 Storage and handing instructions
Store below 25°C. Protected from moisture.
9.0 Patient Counselling Information
- Take this medicine exactly as prescribed by your doctor. Do not change the dose or stop therapy without consulting your doctor.
- Pregnant women and breastfeeding mothers should not use this medicine without doctor consultation.
- Don’t medicine for the treatment of diabetic ketoacidosis or diabetic pre-coma.
- This medicine is not advisable for use in children.
- Avoid use of this medicine during severe infection or before and after surgery or in serious trauma cases.
12.0 Date of revision
10/10/2024
The name of your medicine is ZUVOG. We refer to them as ZUVOG TABLETS or ZUVOG throughout this leaflet.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any more questions, please ask your doctor or your pharmacist. '
- This medicine has been prescribed for you personally and you should not pass it on to anyone else. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects get serious, or if you notice any side effects that are not listed in the leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What ZUVOG Tablets are and what they are used for
2. What you need to know before you take ZUVOG Tablets
3. How to take ZUVOG Tablets
4. Possible side effects
5. How to store ZUVOG Tablets
6. Contents of the pack and other information
1. What ZUVOG Tablets are and What they are used for
Zuvog contains the active substance Voglibose, which is used to control postprandial (after-meal) blood sugar levels in patients with type 2 diabetes mellitus. It is prescribed when diet, exercise, or other medications do not adequately control blood sugar levels.
Voglibose works by blocking certain enzymes in your small intestine that break down complex sugars into simple sugars like glucose. By doing this, it slows down the absorption of sugar into your bloodstream after meals, helping to control blood sugar levels.
2. What You Need to Know Before You Take Zuvog Tablets
Do not take Zuvog if you:
- Are allergic to Voglibose or any of the other ingredients of this medicine.
- Have inflammatory bowel disease, gastrointestinal obstruction, or conditions that may deteriorate due to increased gas formation.
- Have severe ketosis, diabetic coma or pre-coma, severe infection, or hepatic impairment.
Taking other medicines and ZUVOG
- Antidiabetic Medications: Co-administration with sulfonylureas or insulin can enhance the hypoglycemic effect, increasing the risk of hypoglycemia. Close monitoring of blood glucose levels is recommended.
- Beta Blockers: These medications can mask the symptoms of hypoglycemia, making it harder to recognize low blood sugar levels.
- Keratolytics and MAOIs: These can enhance the hypoglycemic effect of Voglibose.
- Anti-lipidemic Agents: These can enhance the hypoglycemic effect of Voglibose.
- Sympathomimetics or Adrenergic Agonists: These can reduce the hypoglycemic effect of Voglibose.
- Adrenocorticotropic and Thyroid Hormones: These can reduce the hypoglycemic effect of Voglibose.
Pregnancy
Voglibose is generally not recommended during pregnancy due to insufficient well-controlled studies in pregnant women. It should only be used if specifically advised by a healthcare professional in rare, carefully evaluated situations.
Breast-feeding
The use of Voglibose during breastfeeding is not well-studied, and there is limited data available on its safety for nursing mothers and infants. It is generally recommended to avoid Voglibose during breastfeeding unless absolutely necessary.
Driving and using machines
Voglibose does not usually affect your ability to drive or use machines. However, if you experience symptoms of hypoglycemia, such as dizziness or confusion, avoid these activities until you feel better.
3. How to Take Efnocar Tablets
Dosage:
The usual dose for adults: One tablet of 0.2 mg three times daily before meals. In some cases,
the dose may be increased to 0.3 mg three times daily.
For type 1 diabetes, Voglibose is administered with insulin.
Method of administration:
Take Zuvog immediately before meals to slow the absorption of carbohydrates and prevent sudden spikes in blood sugar.
If you take more ZUVOG Tablets than you should
Taking more Zuvog Tablets than prescribed can lead to an overdose. While Voglibose is unlikely to cause hypoglycemia (low blood sugar) in overdose, it can cause significant gastrointestinal discomfort. Abdominal discomfort, diarrhea, Flatulence and Bloating may occur. Do not panic. Stay calm and seek medical advice immediately. Contact your doctor or go to the nearest hospital emergency department. Take the medicine packaging with you to show the healthcare professionals. Treatment is generally supportive and symptomatic, focusing on relieving gastrointestinal symptoms.
If you forget to take Zuvog Tablets
If you forget to take a tablet, take one as soon as you remember, unless it is nearly time to take the next one. Never take two doses together. Take the remaining doses at the correct time.
If you stop taking Zuvog Tablets
Take this medicine for as long as your doctor tells you to, as you may become unwell if you stop.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects: Gastrointestinal issues such as flatulence, abdominal distension, diarrhea, and abdominal pain.
Serious side effects:
Hypoglycemia, especially when taken with other anti-diabetic medications.
Hepatotoxicity and skin reactions.
Tell your doctor or pharmacist if you notice any other effects not listed.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top t end of the home page.
Website link: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to Store Efnocar Tablets
Do not use the tablets after the end of the expiry month (use-by date) shown on the product packaging.
Store below 25°C. Protected from moisture.
Keep this medicine out of the sight and reach of children
6. Contents of the Pack and Other Information
What ZUVOG Tablets contain
The active substance is Voglibose
Each tablet contains 0.2/0.3 mg of Voglibose.
Packaging: 10 Blister strips of 10 tablets each