Zuvog Trio 2 Tablet
Therapy Area
Anti-diabetic
1.0 Generic Name
Voglibose, Glimepiride & Metformin Hydrochloride (SR) tablets
2.0 Qualitative and quantitative composition
Zuvog ® Trio-1
Each uncoated bilayered tablet contains:
Voglibose IP........................................... 0.2mg
Glimepiride IP..........................................1mg
Metformin hydrochloride IP.................... 500mg
(in sustained release form)
Excipients q.s.
Colours: Quinoline Yellow Lake and Brilliant Blue
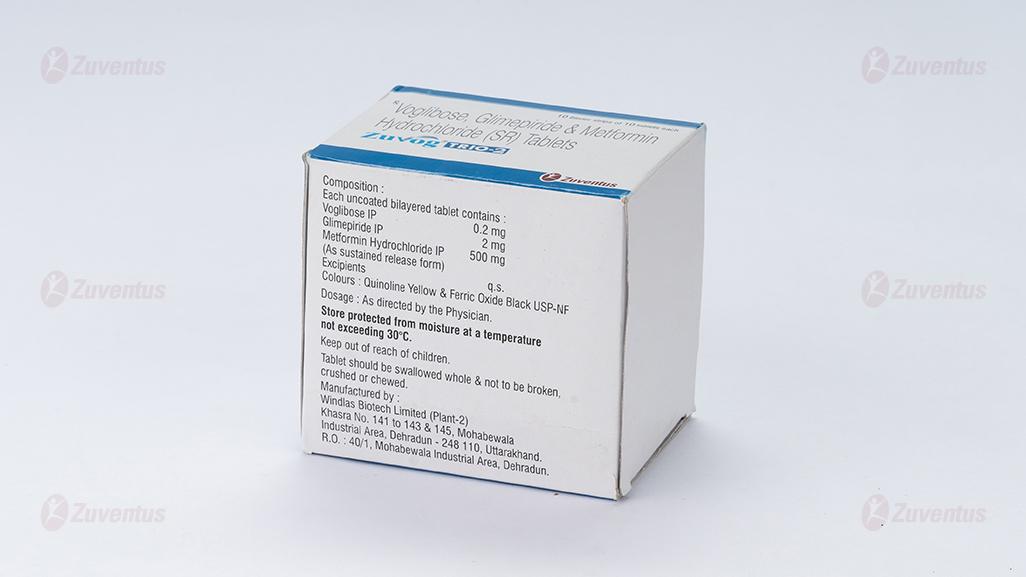
Zuvog ® Trio-2
Each uncoated bilayered tablet contains:
Voglibose IP................................................ 0.2mg
Glimepiride IP............................................... 2mg
Metformin hydrochloride IP
(in sustained release form) ...........................500mg
Excipients q.s.
Colours: Quinoline Yellow Lake and Brilliant Blue
3.0 Dosage form and strength
Tablets
Voglibose (0.2mg), Glimepiride IP 1mg/2mg, Metformin 500mg
4.0 Clinical particulars
4.1 Therapeutic Indication
As 3rd line treatment of type 2 diabetes mellitus when diet, exercise and the single agents and second line therapy with two drugs do not result in adequate glycemic control.
4.2 Posology and method of administration
The usual recommended dose for adults is one tablet of Zuvog ® Trio-1/2, twice a day before meals.
Additionally, voglibose tablets may be taken before the remaining meal, as prescribed by the physician.
Special Populations:
Children
Data are insufficient to recommend pediatric use of Zuvog ® Trio-1/2.
Renal impairment
A GFR should be assessed before initiation of treatment with metformin containing products and at least annually thereafter. In patients at increased risk of further progression of renal impairment and in the elderly, renal function should be assessed more frequently, e.g. every 3-6 months. The maximum daily dose of metformin should preferably be divided into 2-3 daily doses. Factors that may increase the risk of lactic acidosis should be reviewed before considering initiation of metformin in patients with GFR<60 mL/min. If no adequate strength of Zuvog trio is available, individual mono components should be used instead of the fixed dose combination.
| GFR ml/min | Metformin | Glimepiride |
| 60-89 | Maximum daily dose is 3000 Mg. Dose reduction may be considered in relation to declining renal function. | The highest recommended dose per day should be 8 mg of glimepiride |
| 45-59 | Maximum daily dose is 2000 mg. The starting dose is at most half of the maximum dose. | |
| 30-44 | Maximum daily dose is 1000 mg. The starting dose is at most half of the maximum dose./td> | |
| ˂30 | Metformin is contraindicated | Change-over to insulin is indicated, not least to achieve optimal metabolic control |
Method of administration
Due to sustained release formulation, Zuvog trio must be swallowed whole and not crushed or chewed.
4.3 Contraindications
- Patients hypersensitive to glimepiride, voglibose, metformin, other sulfonylureas, other sulfonamides, or any of the excipients
- Pregnant women
- Breast-feeding women
- Any type of acute metabolic acidosis (such as lactic acidosis, diabetic ketoacidosis, diabetic pre-coma).
- Severe renal failure (GFR˂ 30ml/min)
- Hepatic insufficiency
- Acute alcohol intoxication, alcoholism
- Severe infections, before or after operation or with severe trauma
- Gastrointestinal obstruction or predisposed to it
- Acute conditions with the potential to alter renal function such as:
- dehydration
- severe infection
- shock
- intravascular administration of iodinated contrast agents
- Acute or chronic disease which may cause tissue hypoxia such as:
- cardiac or respiratory failure
- recent myocardial infarction
- shock
4.4 Special warnings and precautions for use
Warnings
Glimepiride: In exceptional stress situations (e.g. trauma, surgery, febrile infections) blood glucose regulation may deteriorate, and a temporary change to insulin may be necessary to maintain good metabolic control.
For Metformin:
Lactic acidosis: Metformin accumulation occurs at acute worsening of renal function and increases the risk of lactic acidosis. In case of dehydration (severe diarrhoea or vomiting, fever or reduced fluid intake), Metformin should be temporarily discontinued and contact with a health care professional is recommended. Medicinal products that can acutely impair renal function (such as antihypertensives, diuretics and NSAIDs) should be initiated with caution in metformin-treated patients. Diagnosis: Patients and/or care-givers should be informed of the risk of lactic acidosis. In case of suspected symptoms, the patient should stop taking metformin and seek immediate medical attention Diagnostic laboratory findings are decreased blood pH (˂7.35), plasma lactate levels (˃5 mmol/L), and an increased anion gap and lactate/pyruvate ratio.
Precautions
Zuvog trio should be administered carefully in patients who are receiving other antidiabetic drugs because hypoglycemia may occur.
For Glimepiride:
In the initial weeks of treatment, the risk of hypoglycaemia may be increased and necessitates especially careful monitoring. Factors favouring hypoglycaemia include:
- unwillingness or (more commonly in older patients) incapacity of the patient to cooperate.
- under nourishment, irregular mealtimes or skipped meals.
- imbalance between physical exertion and carbohydrate intake.
- alterations of diet.
- consumption of alcohol, especially in combination with skipped meals.
- impaired renal function.
- severe impairment of liver function.
- overdosage with glimepiride.
- certain uncompensated disorders of the endocrine system affecting carbohydrate metabolism or counter-regulation of hypoglycaemia (as for example in certain disorders of thyroid function and in anterior pituitary or cortico-adrenal insufficiency).
- concurrent administration of certain other medicines.
- treatment with glimepiride in the absence of any indication.
If such risk factors for hypoglycaemia are present, it may be necessary to adjust the dosage of glimepiride or the entire therapy. This also applies whenever illness occurs during therapy or the patient's life-style changes. Those symptoms of hypoglycaemia which reflect the body's adrenergic counter regulation may be milder or absent where hypoglycaemia develops gradually, in the elderly, and where there is autonomic neuropathy or where the patient is receiving concurrent treatment with beta-blockers, clonidine, reserpine, guanethidine or other sympatholytic drugs. Hypoglycaemia can almost always be promptly controlled by immediate intake of carbohydrates (glucose or sugar).
It is known from other sulfonylureas that, despite initially successful counter measures, hypoglycaemia may recur. Patients must, therefore, remain under close observation. Severe hypoglycaemia further requires immediate treatment and follow-up by a physician and, in some circumstances, in-patient hospital care. Treatment of patients with G6PD-deficiency with sulfonylurea agents can lead to haemolytic anaemia. Since glimepiride belongs to the class of sulfonylurea agents, caution should be used in patients with G6PD-deficiency and a non-sulfonylurea alternative should be considered.
For Metformin:
Regular monitoring of thyroid-stimulating hormone (TSH) levels is recommended in patients with hypothyroidism. Long-term treatment with metformin has been associated with a decrease in vitamin B12 serum levels which may cause peripheral neuropathy. Monitoring of the vitamin B12 level is recommended.
Other precautions:
- All patients should continue their diet with a regular distribution of carbohydrate intake during the day. Overweight patients should continue their energy-restricted diet.
- The usual laboratory tests for diabetes monitoring should be performed regularly.
- Metformin alone never causes hypoglycaemia, although caution is advised when it is used in combination with insulin or sulfonylureas.
Voglibose:
Voglibose should be administered with caution to the following patients: patients with history of laparotomy or ileus; patients with chronic intestinal disease accompanied by disturbance in digestion and absorption; patients with aggravating symptoms due to increased generation of intestinal gas (eg, Roemheld syndrome, severe hernia, and stenosis and ulcer of the large intestine) and patients with serious hepatic or renal disorders.
Other precautions
- All patients should continue their dietary restriction with a regular distribution of carbohydrate intake during the day. Overweight patients should continue their energy restricted diet.
- The usual laboratory tests for diabetes monitoring should be performed regularly.
- Patients should be instructed and explained to recognize hypoglycemic symptoms and its management.
- When patients with diabetes are exposed to unusual stress such as fever, trauma, infection, or surgery, a temporary loss of control of blood glucose may occur. At such times insulin therapy may be necessary for some time.
4.5 Drugs interactions
For Glimepiride:
Based on experience with glimepiride and on what is known of other sulfonylureas, the following interactions must be considered:
Glimepiride is metabolized by cytochrome P450 2C9 (CYP2C9). This should be taken into account when glimepiride is coadministered with inducers (e.g. rifampicin) or inhibitors (e.g. fluconazole) of CYP2C9. Potentiation of the blood-glucose-lowering effect and, thus, in some instances hypoglycaemia may occur when one of the following drugs is taken, for example: insulin and other oral antidiabetics; ACE inhibitors; anabolic steroids and male sex hormones; chloramphenicol; coumarin derivatives; cyclophosphamide; disopyramide; fenfluramine; fenyramidol; fibrates; fluoxetine; guanethidine; ifosfamide; MAO inhibitors; miconazole; fluconazole; paraaminosalicylic acid; pentoxifylline (high dose parenteral); phenylbutazone; azapropazone; oxyphenbutazone; probenecid; quinolones; salicylates; sulfinpyrazone; clarithromycin; sulfonamide antibiotics; tetracyclines; tritoqualine; trofosfamide. Weakening of the blood-glucose-lowering effect and, thus raised blood glucose levels may occur when one of the following drugs is taken, for example: acetazolamide; barbiturates; corticosteroids; diazoxide; diuretics; epinephrine (adrenaline) and other sympathomimetic agents; glucagon; laxatives (after protracted use); nicotinic acid (in high doses); oestrogens and progestogens; phenothiazines; phenytoin; rifampicin; thyroid hormones. H2 receptor antagonists, beta-blockers, clonidine and reserpine may lead to either potentiation or weakening of the blood glucose-lowering effect. Under the influence of sympatholytic drugs such as beta-blockers, clonidine, guanethidine and reserpine, the signs of adrenergic counter-regulation to hypoglycaemia may be reduced or absent. Both acute and chronic alcohol intake may potentiate or weaken the blood glucose-lowering action of glimepiride in an unpredictable fashion. The effect of coumarin derivatives may be potentiated or weakened.
Bile acid sequestrant: Colesevelam binds to glimepiride and reduces glimepiride absorption from the gastro-intestinal tract. No interaction was observed when glimepiride was taken at least 4 hours before colesevelam. Therefore glimepiride should be administered at least 4 hours prior to colesevelam
For Metformin:
Concomitant use not recommended:
Alcohol: Alcohol intoxication, particularly in case of fasting, malnutrition or hepatic insufficiency. Avoid consumption of alcohol and alcohol-containing medications.
Combinations requiring precautions for use:
Some medicinal products can adversely affect renal function which may increase the risk of lactic acidosis, e.g. NSAIDs, including selective cyclo-oxygenase (COX) II inhibitors, ACE inhibitors, angiotensin II receptor antagonists and diuretics, especially loop diuretics. When starting or using such products in combination with metformin, close monitoring of renal function is necessary. Glucocorticoids (systemic and local routes), beta-2-agonists and diuretics have intrinsic hyperglycaemic activity. Inform the patient and perform more frequent blood glucose monitoring, especially at the beginning of treatment. If necessary, adjust the dosage of the antidiabetic drug during therapy with the other drug and upon its discontinuation. ACE-inhibitors may decrease the blood glucose levels. If necessary, adjust the dosage of the antidiabetic drug during therapy with the other drug and upon its discontinuation.
Metformin may decrease the anticoagulant effect of phenprocoumon. Therefore, a close monitoring of the INR is recommended. Levothyroxine can reduce the hypoglycemic effect of metformin. Monitoring of blood glucose levels is recommended, especially when thyroid hormone therapy is initiated or stopped, and the dosage of metformin must be adjusted if necessary.
Organic cation transporters (OCT)
Metformin is a substrate of both transporters OCT1 and OCT2.
Co-administration of metformin with
- Inhibitors of OCT1 (such as verapamil) may reduce efficacy of metformin.
- Inducers of OCT1 (such as rifampicin) may increase gastrointestinal absorption and efficacy of metformin.
- Inhibitors of OCT2 (such as cimetidine, dolutegravir, ranolazine, trimethoprime, vandetanib, isavuconazole) may decrease the renal elimination of metformin and thus lead to an increase in metformin plasma concentration.
- Inhibitors of both OCT1 and OCT2 (such as crizotinib, olaparib) may alter efficacy and renal elimination of metformin.
Caution is therefore advised, especially in patients with renal impairment, when these drugs are co-administered with metformin, as metformin plasma concentration may increase. If needed, dose adjustment of metformin may be considered as OCT inhibitors/inducers may alter the efficacy of metformin
For Voglibose
When voglibose is used in combination with derivative(s) of sulfonylamide, sulfonylurea or biguanide, or with insulin, hypoglycemic symptoms may occur. Therefore, when used in combination with any of these drugs, care should be taken, such as starting the administration at a low dose. Voglibose does not affect the pharmacokinetics of warfarin, hence it can be safely administered along with warfarin.
4.6 Use in special populations
Pregnancy
Zuvog Trio must not be taken during pregnancy. Otherwise there is risk of harm to the child. The patient must change over to insulin during pregnancy.
Lactation
To prevent possible ingestion with the breast milk and possible harm to the child, Zuvog Trio must not be taken by breast-feeding women. If necessary the patient must change over to insulin, or must stop breast-feeding.
4.7 Effects on ability to drive and use machines
Alertness and reactions may be impaired due to hypo- or hyperglycemia, especially when beginning or after altering treatment or when Zuvog-TRIO is not taken regularly. This may, for example, affect the ability to drive or to operate machinery.
4.8 Undesirable effects
Zuvog Trio should be administered carefully in patients who are receiving other antidiabetic drugs because hypoglycemia may occur.
Glimepiride
Metabolism and nutrition disorders: As a result of the blood-glucose-lowering action of glimepiride, hypoglycaemia may occur, which may also be prolonged. Possible symptoms of hypoglycaemia include headache, ravenous hunger, nausea, vomiting, lassitude, sleepiness, disordered sleep, restlessness, aggressiveness, impaired concentration, impaired alertness and reactions, depression, confusion, speech disorders, aphasia, visual disorders, tremor, pareses, sensory disturbances, dizziness, helplessness, loss of self-control, delirium, cerebral convulsions, somnolence and loss of consciousness up to and including coma, shallow respiration and bradycardia. In addition, signs of adrenergic counter-regulation may be present such as sweating, clammy skin, anxiety, tachycardia, hypertension, palpitations, angina pectoris, and cardiac arrhythmias. The clinical picture of a severe hypoglycaemic attack may resemble that of a stroke. The symptoms nearly always subside when hypoglycaemia is corrected; Eye disorders: Especially at the start of treatment, there may be temporary visual impairment due to the change in blood glucose levels. The cause is a temporary alteration in the turgidity and hence the refractive index of the lens, this being dependent on blood glucose level; Gastrointestinal disorders: Occasionally, gastrointestinal symptoms such as nausea, vomiting, sensations of pressure or fulness in the epigastrium, abdominal pain and diarrhoea may occur. In isolated cases, there may be hepatitis, elevation of liver enzyme levels and/or cholestasis and jaundice, which may progress to life-threatening liver failure but can regress after withdrawal of glimepiride, dysgeusia (frequency not known); Blood and lymphatic system disorders: Changes in the blood picture may occur: Rarely, thrombocytopenia and, in isolated cases, leucopenia, haemolytic anaemia, erythrocytopenia, granulocytopenia, agranulocytosis or pancytopenia may develop. Cases of severe thrombocytopenia with platelet count less than 10,000/μl and thrombocytopenic purpura have been reported in post-marketing experience (frequency not known); Skin and subcutaneous tissue disorders:Alopecia (frequency not known); General disorders: Occasionally, allergic or pseudoallergic reactions may occur, e.g. in the form of itching, urticaria or rashes. Such mild reactions may develop into serious reactions with dyspnoea and a fall in blood pressure, sometimes progressing to shock. In the event of urticaria a physician must therefore be notified immediately. In isolated cases, a decrease in serum sodium concentration and allergic vasculitis or hypersensitivity of the skin to light may occur.
For Metformin:
Gastrointestinal symptoms such as nausea, vomiting, diarrhoea, abdominal pain and loss of appetite (>10%) are very common: these occur most frequently during initiation of therapy and resolve spontaneously in most cases. To prevent these gastrointestinal symptoms, it is recommended that metformin be taken in 2 or 3 daily doses during or after meals. A slow increase of the dose may also improve gastrointestinal tolerability.
- Metallic taste (3%) is common.
- Mild erythema has been reported in some hypersensitive individuals. The incidence of such effects is regarded as very rare (<0.01%).
- A decrease of vitamin B12 absorption with decrease of serum levels has been observed in patients treated long-term with metformin and appears generally to be without clinical significance (<0.01%).
- However, cases of peripheral neuropathy in patients with vitamin B12 deficiency have been reported in post-marketing experience (frequency not known) • Lactic acidosis (0.03 cases/1000 patient-years) is very rare.
- Hemolytic anemia (frequency unknown)
- Reduction of thyrotropin level in patients with hypothyroidism (frequency unknown)
- Hypomagnesemia in the context of diarrhea (frequency unknown)
- Encephalopathy (frequency unknown)
- Photosensitivity (frequency unknown)
- Hepatobiliary disorders: Reports of liver function tests abnormalities and hepatitis resolving upon metformin discontinuation
For Voglibose:
Gastrointestinal adverse effects such as diarrhoea, loose stools, abdominal pain, constipation, anorexia, nausea, vomiting, or heartburn may occur with the use of Voglibose. Also abdominal distention, increased flatus, and intestinal obstruction like symptoms due to an increase in intestinal gas, may occur with use of Voglibose.
When Voglibose is administered to patients with serious liver cirrhosis, hyperammonia may worsen with the development of constipation followed by disturbance of consciousness. Elevation of GOT (glutamate oxaloacetate), GPT (glutamatepyruvate transaminase), LDH (lactate dehydrogenase), alpha GPT (alpha glutamate pyruvate) or alkaline phosphatase may infrequently occur. When Voglibose is used in combination with other antidiabetic drugs, hypoglycemia may occur (0.1% to <5%).
Hypersensitivity: Rash and pruritus may rarely occur. In such a case, Voglibose tablets should be discontinued.
Psychoneurologic: Headache may rarely occur.
Hematologic: Anemia; thrombocytopenia, and leucopenia may rarely occur.
Others: Numbness, edema of face, blurred vision, hot flushes, malaise, weakness, hyperkalemia, increased serum amylase, decreased HDL cholesterol, diaphoresis or alopecia, and perspiration.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
4.9 Overdose
For Glimepiride:
Signs and Symptoms: Acute overdosage as well as long-term treatment with too high a dose of glimepiride may lead to severe life-threatening hypoglycaemia. Management: As soon as an overdose of glimepiride has been discovered, a physician must be notified without delay. The patient must immediately take sugar, if possible in the form of glucose, unless a physician has already undertaken responsibility for treating the overdose. Careful monitoring is essential until the physician is confident that the patient is out of danger. It must be remembered that hypoglycaemia may recur after initial recovery. Admission to hospital may sometimes be necessary - even as a precautionary measure. In particular, significant overdoses and severe reactions with signs such as loss of consciousness or other serious neurological disorders are medical emergencies and require immediate treatment and admission to hospital. If, for example, the patient is unconscious, an intravenous injection of concentrated glucose
solution is indicated (for adults starting with 40 ml of 20% solution, for example). Alternatively, in adults, administration of glucagon, e.g. in doses of 0.5 to 1 mg i.v., s.c. or i.m. may be considered. In particular, when treating hypoglycaemia due to accidental intake of glimepiride in infants and young children, the dose of glucose given must be very carefully adjusted in view of the possibility of producing dangerous hyperglycaemia, and must be controlled by close monitoring of blood glucose. Patients who have ingested life-threatening amounts of glimepiride require detoxification (e.g. by gastric lavage and medicinal charcoal).
After acute glucose replacement has been completed it is usually necessary to give an intravenous glucose infusion in lower concentration so as to ensure that the hypoglycaemia does not recur. The patient's blood glucose level should be carefully monitored for at least 24 hours. In severe cases with a protracted course, hypoglycaemia, or the danger of slipping back into hypoglycaemia, may persist for several days.
Metformin: Hypoglycaemia has not been seen with metformin doses of up to 85 g, although lactic acidosis has occurred in such circumstances. High overdose or concomitant risks of metformin may lead to lactic acidosis. Lactic acidosis is a medical emergency and must be treated in hospital. The most effective method to remove lactate and metformin is haemodialysis. Pancreatitis may occur in the context of a metformin overdose
Voglibose: Voglibose competitively and reversibly inhibits the alpha glucosidase enzymes (glucoamylase, sucrase, maltase and isomaltase) in the brush border in the small intestine, which delays the hydrolysis of complex carbohydrates. It is unlikely to produce hypoglycemia in overdose, but abdominal discomfort and diarrhea may occur.
5.0 Pharmacological properties
5.1 Mechanism of Action
The administration of vildagliptin results in a rapid and complete inhibition of DPP-4 activity, resulting in increased fasting and postprandial endogenous levels of the incretin hormones GLP-1 (glucagon-like peptide 1) and GIP (glucose-dependent insulinotropic polypeptide).
5.2 Pharmacodynamic properties
Voglibose
Voglibose is an alpha glucosidase inhibitor which inhibits the activity of alpha glucosidases that catalyse the decomposition of disaccharides into monosaccharides in the intestine, thereby delaying the digestion and absorption of carbohydrates, resulting in improvement of postprandial hyperglycaemia.
Metformin hydrochloride
Metformin improves glucose tolerance in patients with type 2 diabetes (NIDDM), lowering both basal and postprandial plasma glucose. Metformin is not chemically or pharmacologically related to sulphonylureas, thiazolidinediones, or alpha-glucosidase inhibitors. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike sulphonylureas, metformin does not produce hypoglycaemia in either patients with type 2 diabetes or normal subjects and does not cause hyperinsulinaemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
Glimepiride
Glimepiride is an orally active hypoglycaemic substance belonging to the sulphonylurea group. It may be used in non-insulin dependent (type 2) diabetes mellitus.
Glimepiride acts mainly by stimulating insulin release from pancreatic beta cells. As with other sulfonylureas this effect is based on an increase of responsiveness of the pancreatic beta cells to the physiological glucose stimulus. In addition, glimepiride seems to have pronounced extrapancreatic effects also postulated for other sulfonylureas.
5.3 Pharmacokinetic properties
Absorption& Distribution
Voglibose
Voglibose is poorly absorbed after oral doses. Plasma concentrations after oral doses have usually been undetectable. After an 80 mg dose (substantially higher than recommended dose), peak plasma levels of about 20 ng/mL were observed in 1-1.5 hours.
Metformin Hydrochloride
The absolute bioavailability of a metformin 500-mg tablet given under fasting conditions is approximately 50-60%. Studies using single oral doses of metformin 500 mg to 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination. Food decreases the extent of and slightly delays the absorption of metformin, as shown by approximately a 40% lower mean peak plasma concentration (Cmax), a 25% lower area under the plasma concentration versus time curve (AUC), and a 35 minute prolongation of time to peak plasma concentration (Tmax) following administration of a single 850-mg tablet of metformin with food, compared to the same tablet strength administered fasting. The clinical relevance of these decreases is unknown.
The apparent volume of distribution (V/F) of metformin following single oral doses of metformin immediate-release 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins, in contrast to sulphonylureas, which are more than 90% protein bound. Metformin partitions into erythrocytes, most likely as a function of time. At usual clinical doses and dosing schedules of metforminimmediate-release, steady state plasma concentrations of metformin are reached within 24-48 hours and are generally <1 µg/mL.
Glimepiride
The bioavailability of glimepiride after oral administration is complete. Food intake has no relevant influence on absorption, only the absorption rate is slightly diminished. Maximum serum concentrations (Cmax) are reached approx 2.5 hours after oral intake (mean 0.3 μg/ml during multiple dosing of 4 mg/daily) and there is a linear relationship between dose and both Cmax and AUC (area under the time concentration curve).
Glimepiride has a very low distribution volume (approx. 8.8 litres), which is roughly equal to the albumin distribution space, high protein binding (>99%) and a low clearance (approx. 48 ml/min).
Metabolism & Excretion
Voglibose
Voglibose is metabolized negligibly and rapidly excreted. When voglibose tablets were repeatedly administered to healthy male adults in a single dose of 0.2 mg, three times a day, for 7 consecutive days, voglibose was not detected in plasma or urine. Also, on administration of voglibose to 10 healthy male subjects in a single dose of 2 mg, voglibose was not detected in plasma or urine.
Metformin Hydrochloride
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion. Renal clearance of metformin is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Mean dominant serum half-life, which is of relevance for the serum concentrations under multiple-dose conditions, is about 5 to 8 hours. After high doses, slightly longer half-lives were noted.
Glimepiride
After a single dose of radiolabelled glimepiride, 58% of the radioactivity was recovered in the urine, and 35% in the faeces. No unchanged substance was detected in the urine. Two metabolites most probably resulting from hepatic metabolism (major enzyme is CYP2C9) were identified both in urine and faeces: the hydroxy derivative and the carboxy derivative. After oral administration of glimepiride, the terminal half-lives of these metabolites were 3 to 6 and 5 to 6 hours respectively.
Comparison of single and multiple once-daily dosing revealed no significant differences in pharmacokinetics, and the intra individual variability was very low. There was no relevant accumulation.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Not available
7.0 Description
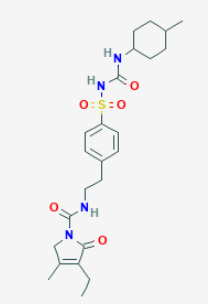
Glimepiride
Glimepiride is a long-acting, third-generation sulfonylurea with hypoglycemic activity. Compared to other generations of sulfonylurea compounds, glimepiride is very potent and has a longer duration of action.
Chemical Name - 4-ethyl-3-methyl-N-[2-[4-[(4 methylcyclohexyl)carbamoylsulfamoyl]phenyl]ethyl]-5-oxo-2Hpyrrole-1-carboxamide
Molecular Weight- 490.6 g/mol
Molecular Formula- C24H34N4O5S
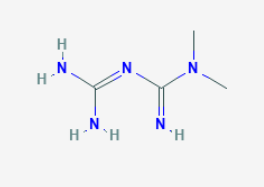
Metformin
Metformin is an agent belonging to the biguanide class of antidiabetics with anti-hyperglycemic activity. Metformin is associated with a very low incidence of lactic acidosis
Chemical Name - 3-(diaminomethylidene)-1,1-dimethylguanidine
Molecular Weight- 129.16 g/mol
Molecular Formula- C4H11N5
Structure-
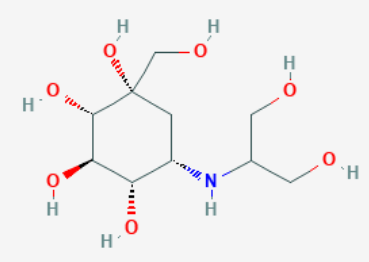
Voglibose
Voglibose is an alpha-glucosidase inhibitor used for lowering post-prandial blood glucose levels in people with diabetes mellitus.
Chemical Name - (1S,2S,3R,4S,5S)-5-(1,3-dihydroxypropan-2-ylamino)-1- (hydroxymethyl)cyclohexane-1,2,3,4-tetrol
Molecular Weight- 267.28 g/mol
Molecular Formula- C10H21NO7
Structure:
8.0 Pharmaceutical particulars
8.1 Incompatibilities
None
8.2 Shelf-life
Refer on pack.
8.3 Packaging information
10 tablets in each blister strip
8.4 Storage and handing instructions
Store protect from moisture, at a temperature not exceeding 25°C.
Keep out of reach of children.
9.0 Patient Counselling Information
Hypoglycemia
Explain the symptoms and treatment of hypoglycemia as well as conditions that predispose to hypoglycemia. Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia and that this may present a risk in situations where these abilities are especially important, such as driving or operating other machinery.
Hypersensitivity Reactions
Inform patients that hypersensitivity reactions may occur with glimepiride tablets and that if a reaction occurs to seek medical treatment and discontinue glimepiride tablets.
Pregnancy
Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy.
Lactation
Advise breastfeeding women taking glimepiride tablets to monitor breastfed infants for signs of hypoglycemia (e.g., jitters, cyanosis, apnea, hypothermia, excessive sleepiness, poor feeding, seizures).
Lactic Acidosis
Explain the risks of lactic acidosis, its symptoms, and conditions that predispose to its development. Advise patients to discontinue metformin hydrochloride extended-release tablets immediately and to promptly notify their healthcare provider if unexplained hyperventilation, myalgias, malaise, unusual somnolence or other nonspecific symptoms occur. Counsel patients against excessive alcohol intake and inform patients about importance of regular testing of renal function while receiving metformin hydrochloride extended-release tablets. Instruct patients to inform their doctor that they are taking metformin hydrochloride extended-release tablets prior to any surgical or radiological procedure, as temporary discontinuation may be required.
Vitamin B12 Deficiency
Inform patients about importance of regular haematological parameters while receiving metformin hydrochloride extended-release tablets.
Females of Reproductive Age:
Inform females that treatment with metformin hydrochloride extended-release tablets may result in ovulation in some premenopausal anovulatory women which may lead to unintended pregnancy.
Metformin SR Tablets Administration Information:
Inform patients that metformin SR tablets must be swallowed whole and not crushed, cut, or chewed, and that the inactive ingredients may occasionally be eliminated in the feces as a soft mass that may resemble the original tablet.
12.0 Date of revision
14/10/2024
About Leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
- What Zuvog® Trio is and what it is used for
- What you need to know before you take Zuvog® Trio
- How to take Zuvog® Trio
- Possible side effects
- How to store Zuvog® Trio
- Contents of the pack and other information
1. What Zuvog® Trio is and what it is used for
Zuvog® Trio contains Voglibose, Glimepiride, and Metformin Hydrochloride (Sustained Release), three different types of medications commonly used to manage type 2 diabetes mellitus.
Voglibose works by inhibiting enzymes (alpha-glucosidases) in the small intestine that break down complex carbohydrates into simple sugars. This slows down the absorption of glucose, thereby reducing postprandial (after meal) blood sugar spikes.
Glimepiride stimulates the release of insulin from pancreatic beta cells. It increases the sensitivity of these cells to glucose, thereby enhancing insulin secretion in response to meals.
Metformin decreases hepatic glucose production, reduces intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. The sustained release formulation allows for a gradual release of the medication, providing a more consistent blood sugar control over time.
Zuvog® Trio, combination of these three medications is used when diet, exercise, and treatment with single or dual therapy do not result in adequate glycemic control. This combination targets different mechanisms to provide a more comprehensive approach to managing blood sugar levels.
2. What you need to know before you take Zuvog® Trio
Do not take Zuvog® Trio if you:
- Are allergic to voglibose, glimepiride, metformin, or any of the other ingredients of this medicine.
- Are pregnant or breastfeeding.
- Have severe kidney or liver problems.
- Have conditions such as lactic acidosis, diabetic ketoacidosis, or severe infections.
Warnings and precautions: Talk to your doctor or pharmacist before taking Zuvog® Trio if you:
- Have kidney or liver problems.
- Are at risk of hypoglycemia (low blood sugar).
- Consume alcohol regularly.
- Are undergoing surgery or radiological procedures.
Children and adolescents: Zuvog® Trio is not recommended for use in children and adolescents.
Other medicines and Zuvog® Trio: Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Some medicines can affect how Zuvog® Trio works or increase the risk of side effects.
Pregnancy and breastfeeding:
Pregnancy: Do not take Zuvog® Trio if you are pregnant. It may harm your unborn baby. If you become pregnant while taking this medicine, inform your doctor immediately.
Breastfeeding: Do not take Zuvog® Trio if you are breastfeeding. It may pass into breast milk and harm your baby. Consult your doctor for alternative treatments.
Driving and using machines:
Zuvog® Trio can cause hypoglycemia (low blood sugar), which may impair your ability to drive or operate machinery. Symptoms of hypoglycemia include dizziness, sweating, and confusion. Do not drive or use machines if you experience these symptoms.
3. How to take Zuvog® Trio
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
- The recommended dose is one tablet twice a day before meals.
- Swallow the tablet whole with water. Do not crush or chew the tablet.
If you take more Zuvog® Trio than you should: Contact your doctor or go to the nearest hospital immediately. Take the medicine pack with you.
If you forget to take Zuvog® Trio: Take it as soon as you remember unless it is almost time for your next dose. Do not take a double dose to make up for a forgotten dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects:
- Nausea, vomiting, diarrhea, abdominal pain, loss of appetite.
Serious side effects:
- Hypoglycemia (low blood sugar): Symptoms include dizziness, sweating, and confusion.
- Lactic acidosis: Symptoms include muscle pain, trouble breathing, stomach pain, dizziness, and feeling cold. Seek medical help immediately if these occur.
Other side effects:
- Headache, dizziness, rash, changes in blood counts.
Reporting of side effects
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top of the home page.
- Website link: https://www.zuventus.com/drug-safety-reporting
- By reporting side effects, you can help provide more information on the safety of this medicine. You can also report the side effect with the help of your treating physician.
5. How to store Zuvog® Trio
- Keep this medicine out of the sight and reach of children.
- Store in a cool, dry place away from moisture and heat.
- Do not use this medicine after the expiry date which is stated on the carton and blister pack.
6. Contents of the pack and other information
What Zuvog® Trio contains:
- The active substances are voglibose, glimepiride, and metformin hydrochloride (sustained release).
Zuvog ® Trio-1
Each uncoated bilayered tablet contains:
Voglibose IP........................................... 0.2mg
Glimepiride IP..........................................1mg
Metformin hydrochloride IP.................... 500mg
(in sustained release form)
Zuvog ® Trio-2
Each uncoated bilayered tablet contains:
Voglibose IP................................................ 0.2mg
Glimepiride IP............................................... 2mg
Metformin hydrochloride IP
(in sustained release form) ...........................500mg
- other ingredients are excipients and coloring agents (Quinoline Yellow Lake and Brilliant Blue).
What Zuvog® Trio looks like and contents of the pack:
- Zuvog® Trio tablets are uncoated bilayered tablets.
- Each pack contains 10 tablets in a blister strip.
Marketing Authorisation Holder and Manufacturer:
Zuventus Healthcare Ltd.
Kamerey Bhasmay, Elaka Pakyong, Rangpo,
East-Sikkim 737 132.
This leaflet was last revised on: 14/10/2024