Eslo 1.25-2.5-5 Tablets
Therapy Area
Cardiology
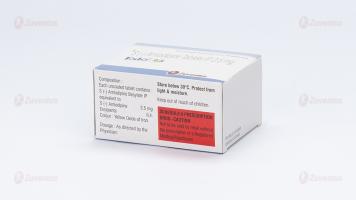
Composition
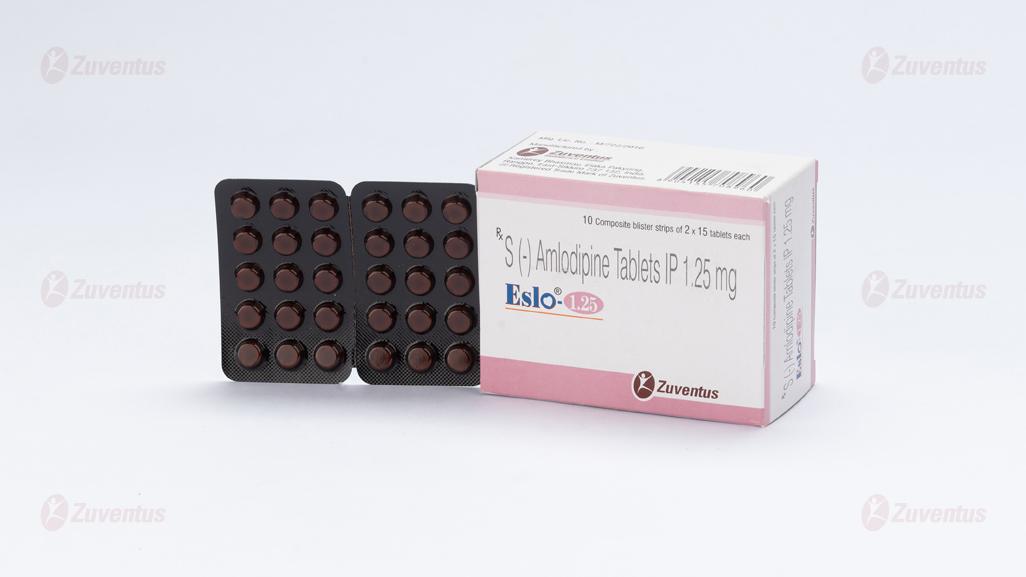
Eslo-1.25
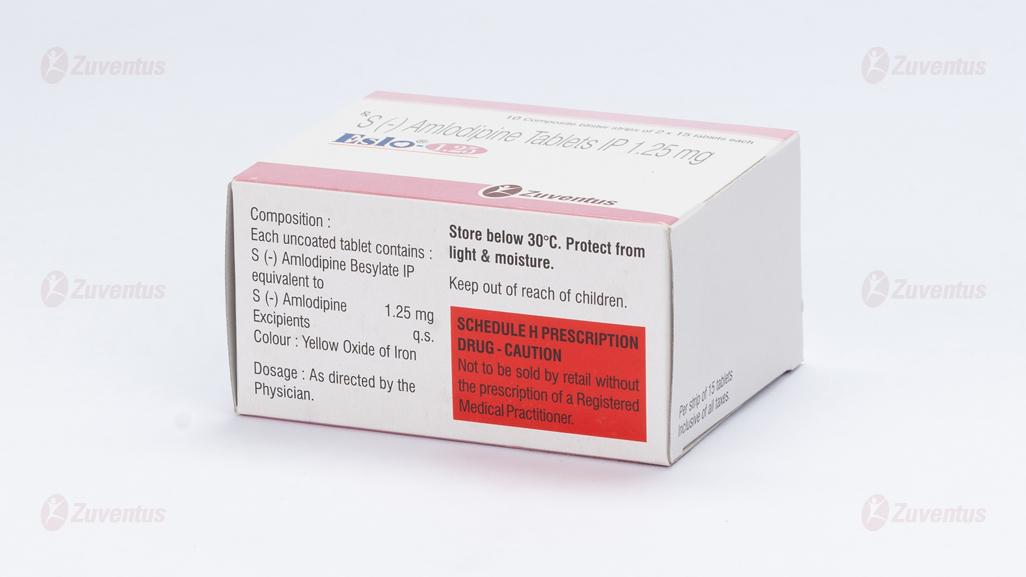
Each uncoated tablet contains :
S (-) Amlodipine Besylate IP
equivalent to S (-) Amlodipine 1.25 mg
Excipients q.s.
Colour : Yellow Oxide of Iron
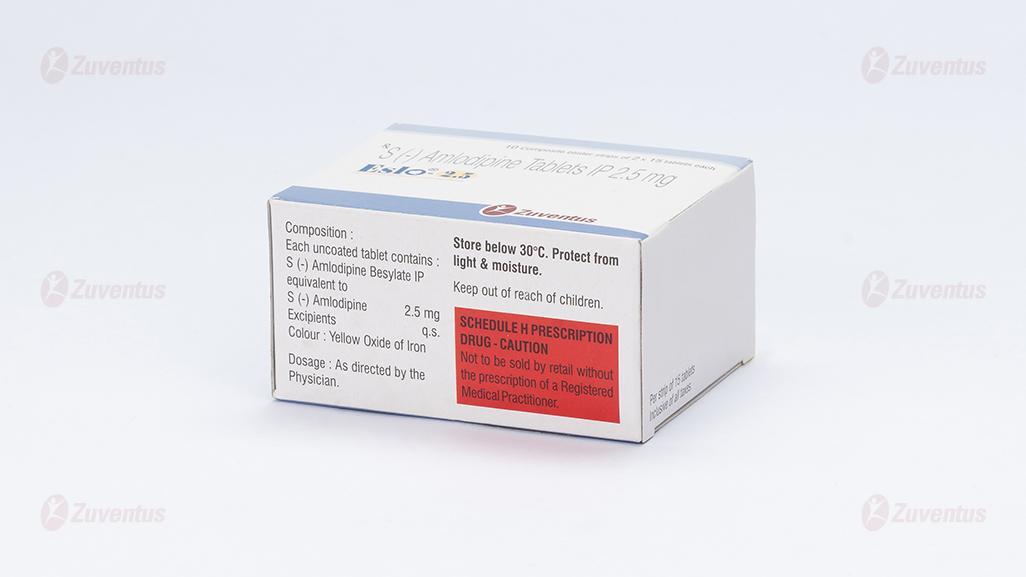
Eslo-2.5
Each uncoated tablet contains :
S (-) Amlodipine Besylate IP
equivalent to S (-) Amlodipine 2.5 mg
Excipients q.s.
Colour : Yellow Oxide of Iron
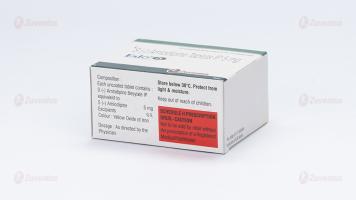
Eslo-5
Each uncoated tablet contains :
S (-) Amlodipine Besylate IP
equivalent to S (-) Amlodipine 5 mg
Excipients q.s.
Colour : Yellow Oxide of Iron
Description
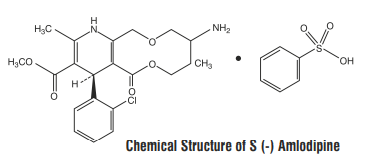
S (-) Amlodipine is the pharmacologically active isomer of Amlodipine.
S (-) Amlodipine is chemically designated as (S)-3-ethyl-5-methyl
2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,
5-pyridinedicarboxylate benzenesulphonate. Its empirical formula
is : C20H25ClN2O5 C6H6O3S with a molecular weight of 567.1
Clinical Pharmacology
Pharmacodynamics
Amlodipine is a racemate with an equal proportion of two enantiomers S and R. The S-enantiomer of Amlodipine is active and the R-enantiomer is inactive in terms of calcium channel blocking activity. S (-) Amlodipine has 1000 fold stronger calcium channel blocking activity than R-Amlodipine. S(-)Amlodipine is therefore responsible for all oftheCCB-mediated pharmacodynamic action ofAmlodipine including its anti-anginal activity. S (-) Amlodipine is a dihydropyridine a calcium channel blocker (CCB) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. S (-) Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle, resulting in reduction in peripheral vascular resistance and reduction in blood pressure. The precise mechanisms by which S (-) Amlodipine relieves angina have not been fully delineated, but are thought to include the following :
- It dilates the peripheral small artery, decreasing peripheral resistance, causing the reduction of energy and oxygen requirement of cardiac muscle.
- It dilates the coronary artery and the small coronary arteries at normal and ischemic areas, increasing the oxygen supply of cardiac muscles of the coronary spasm patients.
Pharmacokinetics and Metabolism
Administration of S (-) Amlodipine 2.5 mg as a single dose in the fasting state produced maximum plasma concentration (Cmax) of 8.30 ± 1.071 ng/ ml in 2.73 ± 0.88 hrs. (Tmax). Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine. Ex vivo studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. The mean AUC value (t= 48 hrs.) 0-t of tablet S (-) Amlodipine (2.5 mg) is 95.33 ± 14.45 ng.hr/ml. The AUC0-∞ value is recorded to be 140.91± 28.06 ng.hr/ml. The plasma elimination half-life of S (-) Amlodipine has been found to be 31.09 ± 12.65 hrs.
Indications
- Essential hypertension
- Angina pectoris
Contraindications
Hypersensitivity to any of the components of the formulation.
Dosage and Administration :
The initial dose is 2.5 mg once a day for the treatment of hypertension and angina pectoris.On the basis of clinical response of patients, the dose may be increased to 5 mg once a day. Elderly individuals or patients with hepatic insufficiency may be started on 1.25 mg once daily. 1.25 mg dose may also be used when titrating up the dose from 2.5 mg to 3.75 mg as per the requirement of the patient.
Adverse Reactions
Gingival hypertrophy and alopecia has been seen with S (-) Amlodipine. On the basis of the clinical data available, only minor side effects have been reported with the use of S (-) Amlodipine. On the basis of clinical data available, adverse events reported in less than 5% of the patients included anxiety (0.43%), anorexia (0.43%), irritation (0.43%), headache (2.13%) and facial flushing (2.13%). Caution should be exercised when administering S (-) Amlodipine to patients with impaired hepatic and renal function.
Drug Interactions
Clinical studies have shown that S (-) Amlodipine when combined with aspirin, nitrates, β-blockers, statins, ACE inhibitors, H receptor 2 blockers and proton pump inhibitors produced no drug interactions.
Precautions :
No controlled clinical study of S (-) Amlodipine has been performed in patients with hepatic impairment and renal impairment. Clinical studies in patients with normal liver function have shown that there is no elevation in the hepatic enzymes with the use of S (-) Amlodipine. However, caution should be taken while administering S (-) Amlodipine to such patients
Pregnant Women & Nursing Mothers
There is no data available on the use of S (-) Amlodipine in pregnant and lactating women, hence the drug should be administered only when the potential benefits outweigh the risk to the patient.
Children
Safety and efficacy of this product in children have not been established.
Overdosage
There are no reported cases of overdosage with the use of S (-) Amlodipine. Overdosage with racemic Amlodipine may cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. Hence, caution should be taken in case of an overdosage with S (-) Amlodipine. If massive overdose occurs, active cardiac and respiratory monitoring should be instituted. Frequent blood pressure measurements should be performed. Should hypotension occur, cardiovascular support including elevation of the extremities and the judicious administration of fluids should be initiated. If hypotension remains unresponsive to these conservative measures, administration of vasopressors (e.g. phenylephrine) should be considered with attention to circulating volume and urine output. If massive overdose occurs, gastric lavage should be employed. As this product is highly plasma protein bound, hemodialysis is not likely to be of benefit.
Storage
Store below 30°C. Protect from light & moisture
Keep out of reach of children
Presentation :
Eslo-1.25 : A composite blister strip of 2 x 15 tablets
Eslo-2.5 : A composite blister strip of 2 x 15 tablets.
Eslo-5 : A composite blister strip of 2 x 15 tablets.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any more questions, please ask your doctor or your pharmacist.
This medicine has been prescribed for you personally and you should not pass it on to anyone else. It may harm them, even if their symptoms are the same as yours.
If any of the side effects get serious, or if you notice any side effects that are not listed in the leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What (S)- Amlodipine Tablets are and what they are used for
2. What you need to know before you take (S)- Amlodipine Tablets
3. How to take (S)- Amlodipine Tablets
4. Possible side effects
5. How to store (S)- Amlodipine Tablets 6. Contents of the pack and other information
1. What Eslo Tablets Are and What They Are Used for
Eslo Tablets contains the active substance (S)- amlodipine besylate which is the pharmacologically active isomer of amlodipine. (S)- amlodipine belongs to a group of medicines called calcium antagonists.
Eslo Tablet is used to treat high blood pressure (hypertension) or a certain type of chest pain called angina, a rare form of which is Prinzmetal’s or variant angina.
In patients with high blood pressure, these medicines work by relaxing blood vessels, so that blood passes through them more easily. In patients with angina, (S)- amlodipine works by improving blood supply to the heart muscle which then receives more oxygen and as a result chest pain is prevented. (S)- amlodipine tablets do not immediately relieve chest pain caused by angina.
2. What You Need to Know Before You Take Eslo Tablets
Do not take Eslo Tablets Tablets if you:
- have ever had an allergic reaction to (S)- amlodipine or any of the ingredients in the tablet listed in the formulation. An allergic reaction may include a rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue.
- have very low blood pressure (hypotension) so that you feel faint or dizzy
- have cardiogenic shock (a condition where your heart cannot pump enough blood for your body’s needs)
- have heart failure due to a heart attack
- have narrowing of the heart valve of the aorta (aortic stenosis)
Take special care with Eslo Tablets
You should inform you doctor if you have or have had any of the following conditions:
- Recent heart attack
- Heart failure
- Liver disease
- You are elderly and your dose needs to be increased
- Severe increase in blood pressure (Hypertensive crisis)
No controlled clinical study of S-amlodipine has been performed in patients with hepatic impairment and renal impairment. Clinical studies in patients with normal liver function have shown that there is no elevation in the hepatic enzymes with the use of S-amlodipine. However, caution should be taken while administering S-amlodipine to such patients.
Other medicines and Amlodipine
Clinical studies have shown that S (-) Amlodipine when combined with aspirin, nitrates, β-blockers, statins, ACE inhibitors, H2 receptor blockers and proton pump inhibitors produced no drug interactions.
Amlodipine with food and drink
The bioavailability of S (-) Amlodipine is not altered by the presence of food and drink.
Pregnant Women & Nursing Mothers
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
There is no data available on the use of Eslo Tablets in pregnant and lactating women, hence the drug should be administered only when the potential benefits outweigh the risk to the patient.
Breast-feeding
It is not known whether (S)- amlodipine is passed into breast milk. If you are breast-feeding or about to start breast-feeding you must tell your doctor before taking Eslo Tablets. Ask your doctor or pharmacist for advice before taking any medicine.
Children
Safety and effectiveness of this product in children have not been established.
Driving and using machines
Taking Eslo Tablets may affect your ability to drive or use machinery because (S)- amlodipine could cause side effects such as dizziness, headaches, nausea or tiredness, all of which could affect your ability to concentrate.
3. How to Take Eslo Tablets
Swallow these tablets with a glass of water at the same time each day. You can take the tablets either before or after meals.
Follow your doctor’s instructions. Check the pharmacy label to see how many tablets to take and how often to take them. If you are still not sure, ask your pharmacist or doctor. The usual doses are described below.
Adults
The initial dose is 2.5 mg once a day for the treatment of hypertension and angina pectoris. On the basis of clinical response of patients, the dose may be increased to 5 mg once a day.
Elderly individuals or patients with hepatic insufficiency may be started on 1.25 mg once daily. 1.25 mg dose may also be used when titrating up the dose from 2.5 mg to 3.75 mg as per the requirement of the patient.
Patients with liver disease
Your doctor may give you a different dose to normal.
If you take more Eslo Tablets than you should
Taking too many tablets may cause your blood pressure to become low or even dangerously low. You may feel dizzy, lightheaded, faint or weak. If blood pressure drop is severe enough shock can occur. Your skin could feel cool and clammy and you could lose consciousness. Excess fluid may accumulate in your lungs (pulmonary oedema) causing shortness of breath that may develop up to 24-48 hours after intake.
Seek immediate medical attention if you take too many Eslo Tablets.
If you forget to take Eslo Tablets
Do not worry. If you forget to take a tablet, leave out that dose completely. Take your next dose at the right time. Do not take a double dose to make up for a forgotten dose.
If you stop taking Eslo Tablets
Your doctor will advise you how long to take this medicine. Your condition may return if you stop using this medicine before you are advised to. It is important to keep taking the tablets. Do not wait until your tablets are finished before seeing your doctor. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
On the basis of the clinical data available, only minor side effects have been reported with the use of Eslo Tablets.
On the basis of clinical data available, adverse events reported in less than 5% of the patients included anxiety (0.43%), anorexia (0.43%), irritation (0.43%), headache (2.13%) and facial flushing (2.13%). Caution should be exercised when administering Eslo Tablets to patients with impaired hepatic and renal function.
Tell your doctor or pharmacist if you notice any other effects not listed.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the tab “Safety Reporting” located on the top end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to Store Eslo Tablets
Do not use the tablets after the end of the expiry month (use-by date) shown on the product packaging. Store in a cool, dry & dark place. Protect from light & moisture. Store in the original package.
KEEP THIS MEDICINE OUT OF THE SIGHT AND REACH OF CHILDREN
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the Pack and Other Information
What Eslo Tablets contains
Eslo-1.25
Each uncoated tablet contains:
S (-) Amlodipine Besylate IP equivalent to S (-) Amlodipine 1.25 mg
Excipients q.s.
Colour: Yellow Oxide of Iron
Eslo-2.5
Each uncoated tablet contains:
S (-) Amlodipine Besylate IP equivalent to S (-) Amlodipine 2.5 mg
Excipients q.s.
Colour: Yellow Oxide of Iron
Eslo-5
Each uncoated tablet contains:
S (-) Amlodipine Besylate IP
equivalent to S (-) Amlodipine 5 mg
Excipients q.s. Colour: Yellow Oxide of Iron
What Eslo Tablets looks like
Eslo-1.25: A composite blister strip of 2 x 15 tablets.
Eslo-2.5: A composite blister strip of 2 x 15 tablets.
Eslo-5: A composite blister strip of 2 x 15 tablets.