Eslo Tan Tab
Therapy Area
Cardiology
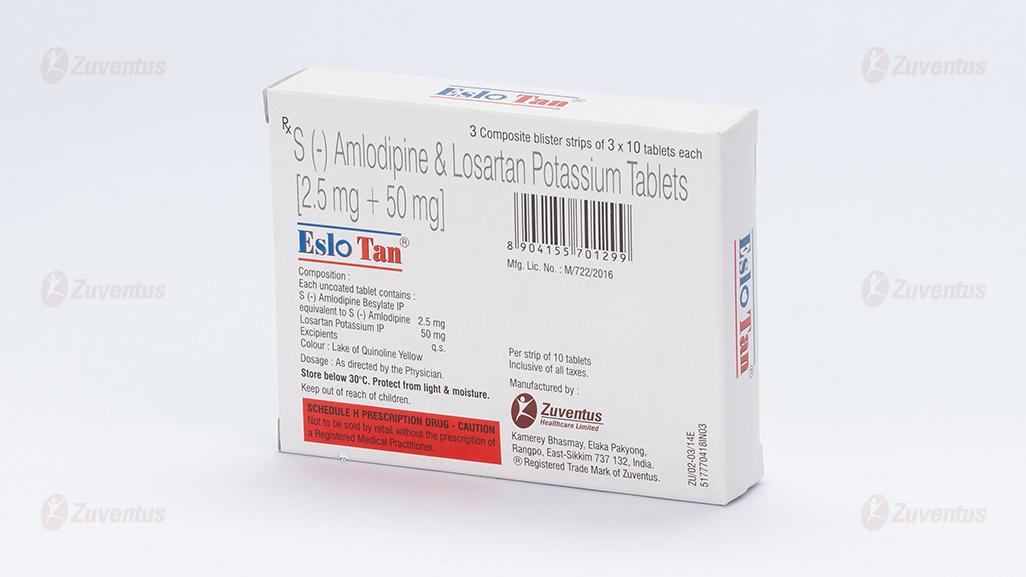
Composition
Each uncoated tablet contains :
S (-) Amlodipine Besylate IP
equivalent to S (-) Amlodipine 2.5 mg
Losartan Potassium IP 50 mg
Excipients q.s.
Colour : Lake of Quinoline Yellow
Description
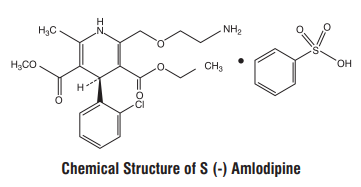
S (-) Amlodipine, the chirally pure form of Amlodipine is a Calcium channel antagonist belonging to the dihydropyridine class. The chemical name of S (-) Amlodipine is (S)-3-ethyl-5-1-methyl-2-(2- aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dih-ydro-6-methyl-3,5-pyridinedicarboxylate. Its empirical formula isC20H25ClN2O5•C6H6O3Swith a molecular weight of 567.1 g/mol
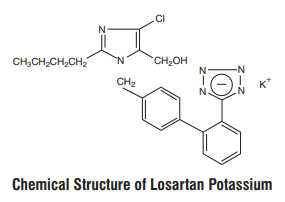
Losartan Potassium, a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o1Htetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium salt. Its empirical formula is C22H22ClKN6O
Clinical Pharmacology :
Pharmacodynamics
Amlodipine is a racemate with an equal proportion of two enantiomers S and R. The S-enantiomer of Amlodipine is active and the R-enantiomer is inactive in terms of calcium channel blocking activity. S (-) Amlodipine has 1000 fold stronger calcium channel blocking activity than R-Amlodipine. S (-) Amlodipine is therefore responsible for all of the CCB-mediated pharmacodynamic action of Amlodipine including its anti-anginal activity. S (-) Amlodipine is a dihydropyridine a calcium channel blocker (CCB) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. S (-) Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle, resulting in reduction in peripheral vascular resistance and reduction in blood pressure. The precise mechanisms by which S (-) Amlodipine relieves angina have not been fully delineated, but are thought to include the following :
- It dilates the peripheral small artery, decreasing peripheral resistance, causing the reduction of energy and oxygen requirement of cardiac muscle
- It dilates the coronary artery and the small coronary arteries at normal and ischemic areas, increasing the oxygen supply of cardiac muscles of the coronary spasm patients
Losartan is a specific and selective antagonist of angiotensin II at AT1 sites. Oxidation of the 5-hydroxymethyl group on the imidazole ring of Losartan during biotransformation results in the formation of the active metabolite E-1374. While Losartan is a competitive antagonist of the AT1 receptor, E-3174 exhibits non-competitive angiotensin II antagonism
Pharmacokinetics
Administration of S (-) Amlodipine 2.5 mg as a single dose in the fasting state produced maximum plasma concentration (Cmax) of 8.30 ± 1.071 ng/ ml in 2.73 ± 0.88 hrs (Tmax). Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine. Ex vivo studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. The mean AUC0-t value (t=48 hrs.) of tablet S (-) Amlodipine (2.5 mg) is 95.33 ± 14.45 ng.hr/ml. The AUC0-∞ value is recorded to be 140.91± 28.06 ng.hr/ml. The plasma elimination half-life of S (-) Amlodipine has been found to be 31.09 ± 12.65 hrs. Following oral administration, Losartan is well absorbed and undergoes first-pass metabolism, forming an active carboxylic acid metabolite and other inactive metabolites. The systemic bioavailability of losartan tablets is approximately 33%. Mean peak concentrations of Losartan and its active metabolite are reached in 1 hour and in 3-4 hours, respectively. Both Losartan and its active metabolite are ≥ 99% bound to plasma proteins, primarily albumin. The volume of distribution of Losartan is 34 litres.
About 14% of an intravenously or orally-administered dose of Losartan is converted to its active metabolite. Following oral and intravenous administration of 14C-labelled Losartan Potassium, circulating plasma radioactivity primarily is attributed to Losartan and its active metabolite. Minimal conversion of losartan to its active metabolite was seen in about one percent of individuals studied. In addition to the active metabolite, inactive metabolites are formed Plasma clearance of losartan and its active metabolite is about 600 ml/min and 50 ml/min, respectively. Renal clearance of Losartan and its active metabolite is about 74 ml/min and 26 ml/min, respectively. When Losartan is administered orally, about 4% of the dose is excreted unchanged in the urine, and about 6% of the dose is excreted in the urine as active metabolite. The pharmacokinetics of losartan and its active metabolite are linear with oral Losartan Potassium doses up to 200 mg. Following oral administration, plasma concentrations of losartan and its active metabolite decline polyexponentially with a terminal half-life of about 2 hours and 6 -9 hours, respectively. During once-daily dosing with 100 mg, neither Losartan nor its active metabolite accumulates significantly in plasma. 14 Both biliary and urinary excretion contribute to the elimination of Losartan and its metabolites. Following an oral dose of C-labelled losartan in man, about 35% / 43% of radioactivity is recovered in the urine and 58% / 50% in the faeces.
Indications
For the treatment of hypertension
Contraindications :
Hypersensitivity to any of the components of the formulation.
Owing to the absence of human data, the combination should not be administered to pregnant women.
Dosage and Administration :
One tablet to be taken orally, once daily.
Adverse Reactions
Gingival hypertrophy and alopecia has been seen with S (-) Amlodipine. On the basis of the clinical data available, only minor side effects have been reported with the use of S (-) Amlodipine. On the basis of clinical data available, adverse events reported in less than 5% of the patients included anxiety (0.43%), anorexia (0.43%), irritation (0.43%), headache (2.13%) and facial flushing (2.13%). Caution should be exercised when administering S (-) Amlodipine to patients with impaired hepatic and renal function.
Common adverse events seen with Losartan are as follows : dizziness, muscle spasm, somnolence, headache, sleep disorders, palpitations, angina pectoris, symptomatic hypotension (especially in patients with intravascular volume depletion, e.g. patients with severe heart failure or under treatment with high dose diuretics), orthostatic hypotension (including dose-related orthostatic effects), rash, abdominal pain, obstipation, vertigo, asthenia, fatigue, oedema, hyperkalemia
Drug Interactions
Clinical studies have shown that S (-) Amlodipine when combined with aspirin, nitrates, beta-blockers, statins, ACE inhibitors, H blockers, and Proton Pump Inhibitors 2 produced no drug interactions. No drug interaction has reported the use of Losartan.
Precautions
Hepatic impairment and renal impairment
No controlled clinical study of S (-) Amlodipine has been performed in patients with hepatic impairment and renal impairment. Clinical studies in patients with normal liver function have shown that there is no elevation in the hepatic enzymes with the use of S (-) Amlodipine. Losartan achieves significantly higher plasma concentrations in cirrhotic patients. Hence, caution should be taken while administering the combination to such patients.
Nursing Mothers
There is no data available on the use of S (-) Amlodipine in lactating women. Atenolol has been successfully used in pregnant patients. Hence, the combination should be administered only when the potential benefits outweighs the risk to the patient.
Children
Safety and effectiveness of this product in children has not been established.
Overdosage
Overdosage with racemic Amlodipine and Losartan may cause excessive peripheral vasodilation with marked hypotension possibly a reflex tachycardia and bradycardia. Hence, caution should be taken in case of an overdosage with Eslo Tan tablet. If massive overdose occurs, active cardiac and respiratory monitoring should be instituted. Frequent blood pressure measurements should be performed. Should hypotension occur, cardiovascular support including elevation of the extremities and the judicious administration of fluids should be initiated. If hypotension remains unresponsive to these conservative measures, administration of vasopressors (such as Phenylephrine) should be considered with attention to circulating volume and urine output. If massive overdose occurs, gastric lavage should be employed. As this product is highly plasma protein bound, hemodialysis is not likely to be of benefit.
Storage
Store below 30°C. Protect from light & moisture.
Keep out of reach of children.
Shelf-life
Refer on the pack
Presentation
PVC/PVDC-Alu blister strip of 10 tablets
About leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you:
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Eslo Tan® is and what it is used for
2. What you need to know before you take Eslo Tan®
3. How to take Eslo Tan®
4. Possible side effects
5. How to store Eslo Tan®
6. Contents of the pack and other information
1. What Eslo Tan® is and what it is used for
Eslo Tan® tablets contain two substances called S(-)Amlodipine and Losartan. Both of these substances help to control high blood pressure.
− S(-)Amlodipine belongs to a group of substances called “calcium channel blockers”. S(-)Amlodipine stops calcium from moving into the blood vessel wall which stops the blood vessels from tightening.
− Losartan belongs to a group of substances called “angiotensin-II receptor antagonists”. Angiotensin II is produced by the body and makes the blood vessels tighten, thus increasing the blood pressure. Losartan works by blocking the effect of angiotensin II.
This means that both substances help stop the blood vessel tightening. As a result, the blood vessels relax and blood pressure is lowered.
Eslo Tan® is used to treat high blood pressure in adults whose blood pressure is not controlled enough with either S(-)Amlodipine or Losartan on its own.
2. What you need to know before you take Eslo Tan®
Do not take Eslo Tan®:
if you are allergic to S(-)Amlodipine, amlodipine, Losartan, any drug of a related class, or any of the other ingredients of this medicine (listed in section 6).
If you think you may be allergic, talk to your doctor before taking Eslo Tan®.
if you have severe liver problems or bile problems such as biliary cirrhosis or cholestasis.
if you are more than 3 months pregnant. (It is also better to avoid Eslo Tan® in early pregnancy, see Pregnancy section).
if you have severe low blood pressure (hypotension).
if you have narrowing of the aortic valve (aortic stenosis) or cardiogenic shock (a condition where your heart is unable to supply enough blood to the body).
if you suffer from heart failure after a heart attack.
if you have diabetes or impaired kidney function and you are treated with a blood pressure lowering medicine containing aliskiren.
If any of the above applies to you, do not take Eslo Tan® and talk to your doctor.
Warnings and precautions
Talk to your doctor before taking Eslo Tan® Tablets:
if you have been sick (vomiting or diarrhoea).
if you have liver or kidney problems.
if you have had a kidney transplant or if you had been told that you have a narrowing of your kidney arteries.
if you have a condition affecting the renal glands called “primary hyperaldosteronism”.
if you have had heart failure or have experienced a heart attack.
Follow your doctor’s instructions for the starting dose carefully.
Your doctor may also check your kidney function.
if your doctor has told you that you have a narrowing of the valves in your heart (called “aortic or mitral stenosis”) or that the thickness of your heart muscle is abnormally increased (called “obstructive hypertrophic cardiomyopathy”).
if you have experienced swelling, particularly of the face and throat while taking other medicines (including angiotensin-converting enzyme inhibitors).
If you get these symptoms, stop taking Eslo Tan® and contact your doctor straight away. You should never take Eslo Tan® again.
if you are taking any of the following medicines used to treat high blood pressure:
- an ACE inhibitor (for example enalapril, lisinopril, ramipril), in particular if you have diabetes-related kidney problems.
- aliskiren.
Your doctor may check your kidney function, blood pressure, and the amount of electrolytes (e.g. potassium) in your blood at regular intervals.
See also information under the heading “Do not take Eslo Tan®”.
If any of these apply to you, tell your doctor before taking Eslo Tan®.
Children and adolescents
The use of Eslo Tan® in children and adolescents is not recommended (aged below 18 years old).
Other medicines and Eslo Tan® Tablets
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Your doctor may need to change your dose and/or to take other precautions. In some cases, you may have to stop taking one of the medicines. This applies especially to the medicines listed below:
Eslo Tan® Tablets may affect or be affected by other medicines, such as:
ACE inhibitors or aliskiren (see also information under the headings “Do not take Eslo Tan ®” and “Warnings and precautions”); diuretics (which increases the amount of urine you produce); lithium (a medicine used to treat some types of depression); potassium-sparing
diuretics, potassium supplements, salt substitutes containing potassium and other substances that may increase potassium levels; certain types of painkillers called non-steroidal anti-inflammatory medicines (NSAIDs) or selective cyclooxygenase-2 inhibitors (COX-2 inhibitors).
Your doctor may also check your kidney function; anticonvulsant agents (e.g. carbamazepine, phenobarbital, phenytoin, fosphenytoin, primidone).
St. John’s wort; nitroglycerin and other nitrates,
or other substances called “vasodilators”;
medicines used for HIV/AIDS (e.g. ritonavir, indinavir, nelfinavir); medicines used to treat fungal infections (e.g. ketoconazole, itraconazole);
medicines used to treat bacterial infections
(such as rifampicin, erythromycin, clarithromycin, telithromycin);
verapamil, diltiazem (heart medicines);
simvastatin (a medicine used to control high cholesterol levels);
dantrolene (infusion for severe body temperature abnormalities);
medicines used to protect against transplant rejection (ciclosporin).
Eslo Tan® Tablets with food and drink
Grapefruit juice and grapefruit should not be consumed by people who are taking Eslo Tan® Tablets. This is because grapefruit and grapefruit juice can lead to an increase in the blood levels of the active ingredient S(-)Amlodipine, which can cause an unpredictable increase in the blood pressure-lowering effect of S(-)Amlodipine.
Pregnancy, breast-feeding and fertility
Pregnancy
You must tell your doctor if you think you are (or might become) pregnant. Your doctor will normally advise you to stop taking Eslo Tan® before you become pregnant or as soon as you know you are pregnant and will advise you to take another medicine instead of Eslo Tan®. Eslo Tan® is not recommended in early pregnancy (first 3 months), and must not be taken when more than 3 months pregnant.
Breast-feeding
Tell your doctor if you are breast-feeding or about to start breast-feeding. Eslo Tan® is not recommended for mothers who are breastfeeding, and your doctor may choose another treatment for you if you wish to breastfeed, especially if your baby is a newborn or was born prematurely. Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
This medicine may make you feel dizzy. This can affect how well you can concentrate. So, if you are not sure how this medicine will affect you, do not drive, use machinery, or do other activities that you need to concentrate on.
3. How to take Eslo Tan® Tablets
Always take or give this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. This will help you get the best results and lower the risk of side effects.
The usual dose of Eslo Tan® is one tablet per day.
− It is preferable to take your medicine at the same time each day.
− Swallow the tablets with a glass of water.
− You can take Eslo Tan® with or without food. Do not take Eslo Tan® with grapefruit or grapefruit juice.
Patients with kidney or liver problems
You should tell your doctor if you have kidney or liver problems as your doctor may need to alter the normal dose or medicine.
If you take more Eslo Tan® than you should
If you have taken too many tablets of Eslo Tan®, or if someone else has taken your tablets, consult a doctor immediately.
If you forget to take or give Eslo Tan®
If you forget to take this medicine, take it as soon as you remember. Then take your next dose at its usual time. However, if it is almost time for your next dose, skip the dose you missed. Do not take a double dose to make up for a forgotten tablet.
If you stop taking Eslo Tan®
Stopping your treatment with Eslo Tan® may cause your disease to get worse. Do not stop taking your medicine unless your doctor tells you to.
4. Possible side effects
This medicine can cause side effects like all medicines, although not everybody gets them.
Some side effects can be serious and need immediate medical attention: A few patients have experienced these serious side effects (may affect up to 1 in 1,000 people). If any of the following happens, tell your doctor straight away: Allergic reaction with symptoms such as rash, itching, swelling of face or lips or tongue, difficulty breathing, low blood pressure (feeling of faintness, light-headedness).
Other possible side effects of Eslo Tan®:
Common (may affect up to 1 in 10 people): blocked nose, sore throat and discomfort when swallowing; headache; swelling of arms, hands, legs, ankles or feet; tiredness; asthenia (weakness); redness and warm feeling of the face and/or neck.
Uncommon (may affect up to 1 in 100 people): Dizziness; nausea and abdominal pain; dry mouth; drowsiness, tingling or numbness of the hands or feet; vertigo; fast heart beat including palpitations; dizziness on standing up; cough; diarrhoea; constipation; skin rash, redness of the skin; joint swelling, back pain; pain in joints.
Rare (may affect up to 1 in 1,000 people): Feeling anxious; ringing in the ears (tinnitus); fainting; passing more urine than normal or feeling more of an urge to pass urine; inability to get or maintain an erection; sensation of heaviness; low blood pressure with symptoms such as dizziness, light-headedness; excessive sweating; skin rash all over your body; itching; muscle spasm.
If any of these affect you severely, tell your doctor.
Side effects reported with S(-)amlodipine or losartan alone and either not observed with Eslo Tan® or observed with a higher frequency than with Eslo Tan®:
S(-)Amlodipine
Consult a doctor immediately if you experience any of the following very rare, severe side effects
after taking this medicine:
- Sudden wheeziness, chest pain, shortness of breath or difficulty in breathing.
- Swelling of eyelids, face or lips.
- Swelling of the tongue and throat which causes great difficulty breathing.
- Severe skin reactions including intense skin rash, hives, reddening of the skin over your whole body, severe itching, blistering, peeling and swelling of the skin, inflammation of the mucous membranes (Stevens-Johnson Syndrome, toxic epidermal necrolysis) or other allergic reactions.
- Heart attack, abnormal heart beat.
- Inflamed pancreas, which may cause severe abdominal and back pain accompanied with feeling of being very unwell.
Losartan
Not known (frequency cannot be estimated from the available data): Decrease in red blood cells, fever, sore throat or mouth sores due to infections; spontaneous bleeding or bruising; high level of potassium in the blood; abnormal liver test results; decreased renal functions and severely decreased renal functions; swelling mainly of the face and the throat; muscle pain; rash, purplish-red spots; fever; itching; allergic reaction; blistering skin (sign of a condition called dermatitis bullous).
If you experience any of these, tell your doctor straight away.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.com and click the “Safety Reporting” located on the top of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to store Eslo Tan®
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the carton and blister. Do not store above 30°C. Store in the original package in order to protect from moisture. Do not use any Eslo Tan® pack that is damaged or shows signs of tampering.
6. Contents of the pack and other information
What Eslo Tan® tablets contain
Eslo Tan® 2.5 mg/50 mg tablets
The active substances of Eslo Tan® are S(-)Amlodipine as s(-) Amlodipine besylate and Losartan potassium. Each tablet contains 2.5 mg S(-)Amlodipine and 50 mg Losartan.
What Eslo Tan® looks like and contents of the pack
Eslo Tan® is available in packs of 10 tablets in PVC/PVDC-Alu blister strips.