4.1. Therapeutic indication
For asthma, rheumatoid arthritis when glucocorticosteriod therapy is warranted.
4.2.Posology and method of administration
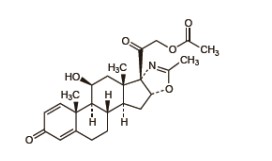
Deflazacort is a glucocorticoid derived from prednisolone and 6mg of deflazacort has approximately the same anti-inflammatory potency as 5mg prednisolone or prednisone. Doses vary widely in different diseases and different patients. In more serious and lifethreatening conditions, high doses of deflazacort may need to be given. When deflazacort is used long term in relatively benign chronic diseases, the maintenance dose should be kept as low as possible. Dosage may need to be increased during periods of stress or in exacerbation of illness. The dosage should be individually titrated according to diagnosis, severity of disease and patient response and tolerance. The lowest dose that will produce an acceptable response should be used.
Adults
For acute disorders, up to 120 mg/day deflazacort may need to be given initially. Maintenance doses in most conditions are within the range 3-18 mg/day. The following regimens are for guidance only
Rheumatoid arthritis: The maintenance dose is usually within the range 3 - 18 mg/day. The smallest effective dose should be used and increased if necessary.
Bronchial asthma: In the treatment of an acute attack, high doses of 48-72 mg/day may be needed depending on severity and gradually reduced once the attack has been controlled. For maintenance in chronic asthma, doses should be titrated to the lowest dose that controls symptoms.
Other conditions: The dose of deflazacort depends on clinical need titrated to the lowest effective dose for maintenance. Starting doses may be estimated on the basis of ratio of 5mg prednisone or prednisolone to 6mg deflazacort.
Hepatic Impairment
In patients with hepatic impairment, blood levels of deflazacort may be increased. Therefore, the dose of deflazacort should be carefully monitored and adjusted to the minimum effective dose.
Renal Impairment
In renally impaired patients, no special precautions other than those usually adopted in patients receiving glucocorticoid therapy are necessary.
Elderly
In elderly patients, no special precautions other than those usually adopted in patients receiving glucocorticoid therapy are necessary. The common adverse effects of systemic corticosteroids may be associated with more serious consequences in old age.
Paediatric Population
There has been limited exposure of children to deflazacort in clinical trials. In children, the indications for glucocorticoids are the same as for adults, but it is important that the lowest effective dosage is used. Alternate day administration may be appropriate.
Doses of deflazacort usually lie in the range 0.25 - 1.5 mg/kg/day. The following ranges provide general guidance:
Juvenile chronic arthritis: The usual maintenance dose is between 0.25 - 1.0 mg/kg/day
Nephrotic syndrome: Initial dose of usually 1.5 mg/kg/day followed by down titration according to clinical need.
Bronchial asthma: On the basis of the potency ratio, the initial dose should be between 0.25 - 1.0 mg/kg deflazacort on alternate days.
Deflazacort withdrawal
In patients who have received more than physiological doses of systemic corticosteroids (approximately 9mg per day or equivalent) for greater than 3 weeks, 3 withdrawal should not be abrupt. How dose reduction should be carried out depends largely on whether the disease is likely to relapse as the dose of systemic corticosteroids is reduced. Clinical assessment of disease activity may be needed during withdrawal. If the disease is unlikely to relapse on withdrawal of systemic corticosteroids but there is uncertainty about HPA suppression, the dose of systemic corticosteroids may be reduced rapidly to physiological doses. Once a daily dose equivalent to 9mg deflazacort is reached, dose reduction should be slower to allow the HPA-axis to recover. Abrupt withdrawal of systemic corticosteroid treatment, which has continued up to 3 weeks is appropriate if it is considered that the disease is unlikely to relapse. Abrupt withdrawal of doses up to 48 mg daily of deflazacort, or equivalent for 3 weeks is unlikely to lead to clinically relevant HPA-axis suppression, in the majority of patients. In the following patient groups, gradual withdrawal of systemic corticosteroid therapy should be considered even after courses lasting 3 weeks or less:
- Patients who have had repeated courses of systemic corticosteroids, particularly if taken for greater than 3 weeks.
- When a short course has been prescribed within one year of cessation of long-term therapy (months or years).
- Patients who may have reasons for adrenocortical insufficiency other than exogenous corticosteroid therapy.
- Patients receiving doses of systemic corticosteroid greater than 48 mg daily of deflazacort (or equivalent),
- Patients repeatedly taking doses in the evening
4.3.Contraindications
Systemic infection unless specific anti-infective therapy is employed. Hypersensitivity to the active substance, deflazacort or any of the excipients. Patients receiving live virus immunisation.
4.4.Special warnings and precautions for use
Patients with rare hereditary problems of galactose intolerance, the Lapp lactose deficiency or glucose-galactose malabsorption should not take this medicine. Undesirable effects may be minimised by using the lowest effective dose for the minimum period, and by administering the daily requirement as a single morning dose or whenever possible as a single morning dose on alternate days. Frequent patient review is required to appropriately titrate the dose against disease activity.
Adrenal suppression
Adrenal cortical atrophy develops during prolonged therapy and may persist for years after stopping treatment. Withdrawal of corticosteroids after prolonged therapy must therefore always be gradual to avoid acute adrenal insufficiency which could be fatal, being tapered off over weeks or months according to the dose and duration of treatment. During prolonged therapy, any intercurrent illness, trauma or surgical procedure will require a temporary increase in dosage; if corticosteroids have been stopped following prolonged therapy, they may need to be temporarily re-introduced. Patients should carry 'Steroid treatment' cards which give clear guidance on the precautions to be taken to minimise risk and which provide details of prescriber, drug, dosage and the duration of treatment.
Anti-inflammatory/immunosuppressive effects and infection
Suppression of the inflammatory response and immune function increases the susceptibility to infections and their severity. The clinical presentation may often be atypical and serious infections such as septicaemia and tuberculosis may be masked and may reach an advanced stage before being recognised. Chickenpox is of particular concern since this normally minor illness may be fatal in immunosuppressed patients. Patients (or parents of children) without a definite history of chicken pox should be advised to avoid close personal contact with chickenpox or herpes zoster and, if exposed, they should seek urgent medical attention. Passive immunisation with varicella zoster immunoglobulin (VZIG) is needed by exposed non-immune patients who are receiving systemic corticosteroids or who have used them within the previous 3 months; this should be given within 10 days of exposure to chickenpox. If a diagnosis of chickenpox is confirmed, the illness warrants specialist care and urgent treatment. Corticosteroids should not be stopped and the dose may need to be increased. Patients should be advised to take particular care to avoid exposure to measles and to seek immediate medical advice if exposure occurs. Prophylaxis with intramuscular normal immunoglobulin may be needed. Live vaccines should not be given to individuals with impaired responsiveness. The antibody response to other vaccines may be diminished.
Visual disturbance
Visual disturbance may be reported with systemic and topical corticosteroid use. If a patient presents with symptoms such as blurred vision or other visual disturbances, the patient should be considered for referral to an ophthalmologist for evaluation of possible causes which may include cataract, glaucoma or rare diseases such as central serous chorioretinopathy (CSCR) which have been reported after use of systemic and topical corticosteroids. Prolonged use of glucocorticoids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves and may enhance the establishment of secondary ocular infections due to fungi or viruses. Use in active tuberculosis should be restricted to those cases of fulminating and disseminated tuberculosis in which deflazacort is used for management with appropriate antituberculosis regimen. If glucocorticoids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged glucocorticoid therapy, these patients should receive chemoprophylaxis. Tendonitis and tendon rupture are known class effect of glucocorticoids. The risk of such reactions may be increased by co-administration of quinolones. Pheochromocytoma crisis, which can be fatal, has been reported after administration of systemic corticosteroids. Corticosteroids should only be administered to patients with suspected or identified pheochromocytoma after an appropriate risk/benefit evaluation.
Special precautions
The following clinical conditions require special caution and frequent patient monitoring is necessary: -
- Cardiac disease or congestive heart failure (except in the presence of active rheumatic carditis), hypertension, thromboembolic disorders. Glucocorticoids can cause salt and water retention and increased excretion of potassium. Dietary salt restriction and potassium supplementation may be necessary.
- Gastritis or oesophagitis, diverticulitis, ulcerative colitis if there is probability of impending perforation, abscess or pyogenic infections, fresh intestinal anastomosis, active or latent peptic ulcer.
- Diabetes mellitus or a family history, osteoporosis, myasthenia gravis, renal insufficiency.
- Emotional instability or psychotic tendency, epilepsy.
- Previous corticosteroid-induced myopathy.
- Liver failure.
- Hypothyroidism and cirrhosis, which may increase glucocorticoid effect.
- Ocular herpes simplex because of possible corneal perforation.
Patients and/or carers should be warned that potentially severe psychiatric adverse reactions may occur with systemic steroids. Symptoms typically emerge within a few days or weeks of starting the treatment. Risks may be higher with high doses/systemic exposure, although dose levels do not allow prediction of the onset, type, severity or duration of reactions. Most reactions recover after either dose reduction or withdrawal, although specific treatment may be necessary. Patients/carers should be encouraged to seek medical advice if worrying psychological symptoms develop, especially if depressed mood or suicidal ideation is suspected. Patients/carers should also be alert to possible psychiatric disturbances that may occur either during or immediately after dose tapering/withdrawal of systemic steroids, although such reactions have been reported infrequently.
Particular care is required when considering the use of systemic corticosteroids in patients with existing or previous history of severe affective disorders in themselves or in their first degree relatives. These would include depressive or manic-depressive illness and previous steroid psychosis. Glucocorticoids are known to cause irregular menstruation and leukocytosis, care should be taken with deflazacort.
Paediatric population
Corticosteroids cause dose-related growth retardation in infancy, childhood and adolescence which may be irreversible. Hypertrophic cardiomyopathy has been reported after systemic administration of glucocorticosteroids in preterm infants. In infants receiving administration of systemic glucocorticosteroids, echocardiograms should be performed to monitor myocardial structure and function.
Use in Elderly
The common adverse effects of systemic corticosteroids may be associated with more serious consequences in old age, especially osteoporosis, hypertension, hypokalaemia, diabetes, susceptibility to infection and thinning of the skin. Close clinical supervision is required to avoid life-threatening reactions. Since complications of glucocorticoid therapy are dependent on dose and duration of therapy, the lowest possible dose must be given and a risk/benefit decision must be made as to whether intermittent therapy should be used.
4.5.Interaction with other medicinal products and other forms of interaction
- The same precautions should be exercised as for other glucocorticoids. Deflazacort is metabolised in the liver. It is recommended to increase the maintenance dose of deflazacort if drugs which are liver enzyme inducers are co-administered, e.g. rifampicin, rifabutin, carbamazepine, phenobarbitone, phenytoin, primidone and aminoglutethimide. For drugs which inhibit liver enzymes, e.g. ketoconazole it may be possible to reduce the maintenance dose of deflazacort.
- In patients taking estrogens, corticosteroid requirements may be reduced.
- The desired effects of hypoglycaemic agents (including insulin), anti-hypertensives and diuretics are antagonised by corticosteroids and the hypokalaemic effects of acetazolamide, loop diuretics, thiazide diuretics, beta 2-agonists, xanthines and carbenoxolone are enhanced.
- The efficacy of coumarin anticoagulants may be enhanced by concurrent corticosteroid therapy and close monitoring of the INR or prothrombin time is required to avoid spontaneous bleeding.
- In patients treated with systemic corticosteroids, use of non-depolarising muscle relaxants can result in prolonged relaxation and acute myopathy. Risk factors for this include prolonged and high dose corticosteroid treatment, and prolonged duration of muscle paralysis. This interaction is more likely following prolonged ventilation (such as in the ITU setting).
- The renal clearance of salicylates is increased by corticosteroids and steroid withdrawal may result in salicylate intoxication.
- As glucocorticoids can suppress the normal responses of the body to attack by micro-organisms, it is important to ensure that any anti-infective therapy is effective and it is recommended to monitor patients closely. Concurrent use of glucocorticoids and oral contraceptives should be closely monitored as plasma levels of glucocorticoids may be increased. This effect may be due to a change in metabolism or binding to serum proteins. Antacids may reduce bioavailability; leave at least 2 hours between administration of deflazacort and antacids. 8
- Co-treatment with CYP3A inhibitors, including cobicistat-containing products, is expected to increase the risk of systemic side-effects. The combination should be avoided unless the benefit outweighs the increased risk of systemic corticosteroid side-effects, in which case patients should be monitored for systemic corticosteroid side-effects.
4.6.Fertility, pregnancy and lactation
Pregnancy
The ability of corticosteroids to cross the placenta varies between individual drugs, however, deflazacort does cross the placenta. Administration of corticosteroids to pregnant animals can cause abnormalities of foetal development including cleft palate, intra-uterine growth retardation and effects on brain growth and development. There is no evidence that corticosteroids result in an increased incidence of congenital abnormalities, such as cleft palate/lip in man. However, when administered for prolonged periods or repeatedly during pregnancy, corticosteroids may increase the risk of intra-uterine growth retardation. Hypoadrenalism may, in theory, occur in the neonate following prenatal exposure to corticosteroids but usually resolves spontaneously following birth and is rarely clinically important. As with all drugs, corticosteroids should only be prescribed when the benefits to the mother and child outweigh the risks. When corticosteroids are essential however, patients with normal pregnancies may be treated as though they were in the non-gravid state.
Breast-feeding
Corticosteroids are excreted in breast milk, although no data are available for deflazacort. Doses of up to 50 mg daily of deflazacort are unlikely to cause systemic effects in the infant. Infants of mothers taking higher doses than this may have a degree of adrenal suppression but the benefits of breast feeding are likely to outweigh any theoretical risk.
Fertility
No data is available on Deflazacort and its effects on fertility.
4.7.Effects on ability to drive and use machines
The effect of corticosteroids on the ability to drive or use machinery has not been systematically evaluated. Vertigo is a possible undesirable effect after treatment with deflazacort. If affected, patients should not drive or operate machinery.
4.8.Undesirable effects
The incidence of predictable undesirable effects, including hypothalamic-pituitaryadrenal suppression correlates with the relative potency of the drug, dosage; timing of administration and the duration of treatment. The following CIOMS frequency rating is used: Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10000 to <1/1000); very rare (<1/10000), not known (cannot be estimated from the available data).
Endocrine disorders
Uncommon: Suppression of the hypothalamic-pituitary-adrenal axis, amenorrhoea, Cushingoid facies. Not known: Growth suppression in infancy, childhood and adolescence.
Metabolism and nutrition disorders
Common: Weight gain. Uncommon: impaired carbohydrate tolerance with increased requirement for antidiabetic therapy, sodium and water retention with hypertension, potassium loss and hypokalaemic alkalosis when co-administered with beta 2-agonist and xanthines. Not known: Negative protein and calcium balance, increased appetite.
Infections and Infestations
Uncommon: Increased susceptibility and severity of infections with suppression of clinical symptoms and signs, opportunistic infections, recurrence of dormant tuberculosis. Not known: candidiasis.
Musculoskeletal and connective tissue disorders
Uncommon: Osteoporosis, vertebral and long bone fractures. Rare: Muscle wasting. Not known: avascular osteonecrosis, tendonitis and tendon rupture when coadministered with quinolones, myopathy (acute myopathy may be precipitated by nondepolarising muscle relaxants, negative nitrogen balance.
Reproductive system and breast disorders
Not known: Menstrual irregularity.
Cardiac disorders
Not known: Heart failure, hypertrophic cardiomyopathy in preterm infants.
Nervous system disorders
Uncommon: Headache, vertigo.
Not known: restlessness, Increased intra-cranial pressure with papilloedema in children (pseudotumour cerebri), usually after treatment withdrawal, aggravation of epilepsy.
Psychiatric disorders
A wide range of psychiatric reactions including affective disorders such as: Uncommon: depressed and labile mood. Not known: irritable, euphoric, suicidal thoughts. Psychotic reactions including: Not known: mania, delusions, hallucinations, aggravation of schizophrenia Other reactions including: Uncommon: behavioural disturbances. Not known: anxiety, sleep disturbances, and cognitive dysfunction including confusion and amnesia have been reported. Reactions are common and may occur in both adults and children. In adults, the frequency of severe reactions has been estimated to be 5-6%. Psychological effects have been reported on withdrawal of corticosteroids; the frequency is unknown.
Eye disorders
Not known: Vision blurred, increased intra-ocular pressure, glaucoma, papilloedema, posterior subcapsular cataracts especially in children, chorioretinopathy, corneal or scleral thinning, exacerbation of ophthalmic viral or fungal diseases.
Gastrointestinal disorders
Uncommon: Dyspepsia, peptic ulceration, haemorrhage, nausea. Not known: perforation of peptic ulcer, acute pancreatitis (especially in children), candidiasis.
Skin and subcutaneous tissue disorders
Uncommon: hirsutism, striae, acne.
Rare: bruising.
Not known: Skin atrophy, telangiectasia.
General disorders and administration site conditions
Uncommon: Oedema.
Not known: impaired healing.
Immune system disorders
Uncommon: Hypersensitivity including anaphylaxis has been reported.
Blood and lymphatic system disorders
Not known: Leukocytosis.
Vascular disorders
Not known: Thromboembolism in particular in patients with underlying conditions associated with increased thrombotic tendency, rare incidence of benign intracranial hypertension.
Withdrawal symptoms and signs
Not known: Too rapid a reduction of corticosteroid dosage following prolonged treatment can lead to acute adrenal insufficiency, hypotension and death. A 'withdrawal syndrome' may also occur including fever, myalgia, arthralgia, rhinitis, conjunctivitis, painful itchy skin nodules and loss of weight. This may occur in patients even without evidence of adrenal insufficiency.
Class effect
Pheochromocytoma crisis has been reported with other systemic corticosteroids and is a known class effect.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com By reporting side effects, you can help provide more information on the safety of this medicine.
4.9. Overdose
It is unlikely that treatment is needed in cases of acute overdosage. The LD50 for the oral dose is greater than 4000 mg/kg in laboratory animals.