Instamune Powder
1.0 Generic name
Bovine Colostrum, Vitamin A, D, E, K & Zinc Powder
2.0 Qualitative and quantitative composition
Each serve (3 g) of INSTAMUNE® provides:
Bovine Colostrum (with 30% IgG)-1000 mg Zinc-3.3 mg
Vitamin K-10 mcg
Vitamin A-1200 IU
Vitamin D-90 IU
Vitamin E-4 IU
3.0 Dosage form and strength
Powder
90 grams per pack
4.0 Clinical Particulars
4.1 Therapeutic indication
For children & adults to boost immunity.
4.2 Posology and method of administration
Recommended usage level:
Take one spoonful (3 g) with ½ cup of Milk/Water.
4.3 Contraindications
Known allergy to bovine colostrum or any of the ingredient of this product.
4.4 Special warnings and precautions for use
- Allergen Information: Product may contain Milk.
- This product shall be given only under medical advice by Physician / Certified Dietician / Nutritionist.
- This product is not to be used as a substitute for a varied diet.
- This product is not intended to diagnose, treat, cure or prevent any disease.
4.5 Drugs interactions
Bovine colostrum is generally well-tolerated, and no significant herb or drug interactions have been widely reported.
However, individuals with a known allergy to cow’s milk should avoid it.
4.6 Use in special populations
General Precautions: Due to the lack of comprehensive studies, it’s generally recommended to be cautious and it’s essential to consult with a healthcare provider before incorporating bovine colostrum into your diet during pregnancy or lactation.
4.7 Effects on ability to drive and use machines
There is no specific evidence to suggest that bovine colostrum has any direct effects on driving ability or use of machines. No effect anticipated.
4.8 Undesirable effects
- Colostrum is a high-protein, low fat and reduced lactose dairy product, as a result lactose-intolerant individuals generally experience no problems and some even report that colostrum helps with their condition. Colostrum used naturally contains low levels of lactose and during processing these are reduced further. If you can drink milk without any problem, then you can probably take colostrum.
- Some individuals may experience a mild gastrointestinal distress or a bloated feeling, which can be avoided by lowering the dose.
- If this reaction continues however then it is best to discontinue the colostrum treatment. However, as with all health supplements, it is always advisable to consult with your doctor in the first instance.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to: medico@zuventus.com
Website: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
- Not to exceed the recommended daily usage.
- Overdosing on bovine colostrum is not well-documented, but taking it in excessive amounts could potentially lead to some side effects. Gastrointestinal Issues: High doses of bovine colostrum might cause nausea, bloating, diarrhea, or other digestive discomforts.
- In case of accidental overdose, contact a Healthcare Professional.
5.0 Pharmacological Properties
5.1 Pharmacodynamic properties
Bovine colostrum is a nutrient-rich fluid produced by cows in the first few days after giving birth. It’s packed with proteins, antibodies, and growth factors that help newborn calves thrive. For humans, bovine colostrum is often used in supplements due to its potential health benefits, such as boosting immunity, fighting infections, and promoting gut health.
This “early” milk has a nutrient profile and immunological composition significantly different from “mature” milk. In addition to macronutrients found in milk such as protein, carbohydrate, and fat, and micronutrients including vitamins and minerals, BC contain:
–Oligosaccharides,
–Growth factors,
– Antimicrobial compounds, &
– Immune-regulating constituents
either not present in milk or present in substantially lower concentrations.
Colostrum supports the human in two main ways.
1. Its multiple immune factors and natural antibiotics provide strong support for the immune system.
2.Its many growth factors offer a broad- spectrum boost to encourage optimum health and healing.
- The immunoglobulin, growth factors, antibodies play a role in prevention of infection that is in passive immunity.
- The vital nutrients help for tissue development, growth and energy.
- The growth factors present in the colostrum provide a novel treatment option for gastrointestinal conditions.
5.3 Pharmacokinetic properties
- Colostrum survives human GIT & works on mucosal surfaces: Bovine colostrum contains special glycoproteins and protease inhibitors which are extremely effective at protecting the destruction of colostrum’s active components (immunoglobulins and growth factors) by adult human digestive acids pancreatic enzymes and human stomach acids. This allows them to remain active as they pass into the bowel.
- The major benefit of immune factors from colostrum was shown to be their protective activity in the intestine on the walls of the bowel, and bronchials, (mucosal surfaces).
- The preservation of the biological activity of IgG (immunoglobulin) in the digestive secretions of adults receiving bovine immune colostrum orally indicates – passive enteral (intestinal) immunization for the prevention and treatment of acute intestinal diseases.
- Bovine colostrum has been shown to be safe, natural, effective & biologically transferable for human use.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
Not available
7.0 Description
Bovine Colostrum: Universal Donor of Colostrum to Human
- Only bovine colostrum has been shown to be safe, natural, effective & biologically transferable for human use.
- Colostrum is a nontoxic, non-allergenic food supplement that has no negative interactions with drugs, food, or other supplements.
- Bovine colostrum is not a drug: It is a safe, natural, non-allergenic food, taken by humans to treat and heal different conditions
- Help create new levels of vitality & well-being.
8.0 Pharmaceutical Properties
8.1 Incompatibilities
Not Applicable
8.2 Shelf-life
Refer on pack
8.3 Packaging information
Pack net weight:90 g Approximately Serving Size: 1 (3 g) No. of servings per pack: 30
8.4 Storage and handing instructions
Store below 30°C.
Protect from moisture.
Product is required to be stored out of reach of children.
9.0 Patient Counselling Information
- NOT FOR MEDICINAL USE.
- Not to be used as a substitute for a varied diet.
- Not intended to diagnose, treat, cure or prevent any disease.
- This product shall be given only under medical advice by Physician / Certified Dietician / Nutritionist.
- Not to exceed the recommended daily usage.
12.0 Date of revision of the text
25th September 2024
Please read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1. What INSTAMUNE® is and what it is used for
2. What you need to know before you use INSTAMUNE®
3. How to use INSTAMUNE®
4. Possible side effects
5. How to store INSTAMUNE®
6. Contents of the pack and other information
1. What INSTAMUNE® is and what it is used for
INSTAMUNE® is a dietary supplement containing bovine colostrum, vitamins A, D, E, K, and zinc. Colostrum is the milk produced during the first few days after birth and contains high levels of immunoglobulins, antimicrobial peptides, and growth factors. Colostrum is important for supporting the growth, development, and immunologic defence of neonates.
It is used to boost immunity in both children and adults.
2. What you need to know before you take INSTAMUNE®
Do not take INSTAMUNE® if:
You are allergic to bovine colostrum or any of the ingredients in this product. Warnings and precautions:
This product may contain milk. Consult your physician, certified dietician, or nutritionist before using this product.
This product is not a substitute for a varied diet.
This product is not intended to diagnose, treat, cure, or prevent any disease.
In case of overdosage:
- Not to exceed the recommended daily usage.
- In case of accidental overdose, contact a Healthcare Professional
Pregnancy and lactation:
Pregnancy: Due to the lack of comprehensive studies, it is generally recommended to be cautious. Consult with a healthcare provider before incorporating bovine colostrum into your diet during pregnancy.
Lactation: Similar precautions apply during breastfeeding. Consult with a healthcare provider to ensure safety.
Driving and using machines:
There is no specific evidence to suggest that bovine colostrum has any direct effects on driving ability or the use of machines. No effect is anticipated.
3. How to take INSTAMUNE®
Recommended dosage:
Take one spoonful (3 g) with ½ cup of milk or water.
4. Possible side effects
Like all supplements, this product can cause side effects, although not everybody gets them. Some individuals may experience mild gastrointestinal distress or a bloated feeling. If these symptoms persist, discontinue use and consult your doctor.
Reporting of side effects: If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.You can also report side effects directly: Website: www.zuventus.com in and click the tab “Safety Reporting” located on the top end of the home page.
Website link: https://www.zuventus.co.in/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this product.
5. How to store INSTAMUNE®
Store below 30°C.
Protect from moisture.
Keep out of reach of children.
6. Contents of the pack and other information
What INSTAMUNE® contains:
Bovine Colostrum (with 30% IgG) - 1000 mg
Zinc - 3.3 mg Vitamin K - 10 mcg
Vitamin A - 1200 IU
Vitamin D - 90 IU
Vitamin E - 4 IU
Pack size: 90 grams per pack (approximately 30 servings)
Manufacturer: Premier Nutraceuticals Pvt. Ltd.,
Dehradun, Uttarakhand, India
Marketed by: Zuventus Healthcare Ltd.,
Mumbai, Maharashtra, India
Last revision date: 25th September 2024.
For More Information About This Product
Feronia HP
1.0 Generic Name
Ferrous ascorbate, Folic Acid, Methylcobalamin and Zinc sulphate
2.0 Qualitative and Quantitative Composition
Each Film-coated tablet contains:
Ferrous ascorbate equivalent to Elemental iron 100mg
Folic Acid IP 1.5mg
Methylcobalamin IP 1.5 mg
Zinc sulphate monohydrate eq. 22.5 mg
to Elemental Zinc
Excipients q.s.
Colour: Red Oxide of Iron and Titanium Dioxide IP
3.0 Dosage Form and Strength
Film coated tablet
Ferrous Ascorbate 100 mg, Folic Acid 1.5 mg, Methylcobalamin 1.5 mg, Zinc Sulphate 22.5mg
4.0 Clinical particulars
4.1 Therapeutic indications
For the treatment of iron deficiency anemia.
4.2 Posology and method of administration
Adults
One tablet should be taken once daily by mouth.
Pediatric Population
There is no relevant use of Feronia HP Tablets in the pediatric population.
4.3 Contraindications
- Hypersensitivity to the active substances or any of the excipients.
- Paroxysmal nocturnal hemoglobinuria, hemosiderosis, haemochromatosis, active peptic ulcer, repeated blood transfusion, regional enteritis and ulcerative colitis.
- Feronia HP Tablets must not be used in the treatment of anemias other than those due to iron deficiency.
- Copper deficiency: Zinc may inhibit the absorption of Copper
4.4 Special warnings and precautions for use
CAUTION: Tablets are hygroscopic in nature. Exposure to humid conditions may soften the tablets affecting their appearance and shape. Cutting of strip should be avoided while dispensing/usage.
The label will state “Important warning: Contains Iron. Keep out of reach and sight of children, as overdose may be fatal”.
This will appear on the front of the pack within a rectangle in which there is no other information.
Some post-gastrectomy patients show poor absorption of iron. Care is needed when treating iron deficiency anemia in patients with treated or controlled peptic ulceration. Caution should be exercised when administering folic acid to patients who may have folate dependent tumors.
Since anemia due to combined iron and vitamin B12 or folate deficiencies may be microcytic in type, patients with microcytic anemia resistant to therapy with iron alone should be screened for vitamin B12 or folate deficiency.
Accumulation of zinc may occur in cases of renal failure.
Pregnancy
Safety is not established in pregnant women; therefore Feronia HP is not recommended in pregnancy.
Lactation
Safety is not established in lactating women; therefore Feronia HP is not recommended during lactation.
Pediatric population
Feronia HP Tablets should be kept out of the reach of children.
4.5 Interaction with other medicinal products and other forms of interaction
- Iron reduces the absorption of penicillamine. Iron compounds impair the bioavailability of fluoroquinolones, levodopa, carbidopa, thyroxine and bisphosphonates.
- Absorption of both iron and antibiotic may be reduced if Feronia HP is given with tetracycline.
- Concurrent administration of antacids may reduce absorption of iron. Co- trimoxazole, chloramphenicol, sulphasalazine, aminopterin, methotrexate, pyrimethamine or sulphonamides may interfere with folate metabolism. Serum levels of anticonvulsant drugs may be reduced by administration of folate.
- Oral chloramphenicol delays plasma iron clearance, incorporation of iron into red blood cells and interferes with erythropoiesis.
- Some inhibition of iron absorption may occur if it is taken with cholestyramine, trientine, tea, eggs or milk.
- Administration of oral iron may increase blood pressure in patients receiving methyldopa.
- Coffee may be a factor in reducing iron bioavailability. Neomycin may alter the absorption of iron.
- The absorption of zinc may be reduced by calcium supplements, tetracyclines and phosphorus-containing compounds, agents which increase gastric pH, such as H2 blockers, may decrease zinc absorption while zinc may reduce the absorption of penicillamine, tetracyclines, fluoroquinolones.
Food
Studies of the co-administration of zinc with food performed in healthy volunteers showed that the absorption of zinc was significantly delayed by many foods (including bread, hard boiled eggs, coffee and milk). Substances in food, especially phytates and fibres, bind zinc and prevent it from entering the intestinal cells. However, protein appears to interfere the least.
4.6 Fertility, pregnancy and lactation
Pregnancy
Safety is not established in pregnant women; therefore Feronia HP is not recommended in pregnancy. Breast-feeding Safety is not established in lactating women; therefore Feronia HP is not recommended during lactation. Fertility No fertility data is available.
4.7 Effects on ability to drive and use machines
Feronia HP Tablets has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Very rare (<1/10,000)
Very rare (<1/10,000):
Rarely allergic reactions may occur.
Not known (cannot be estimated from the available data)
Not known: Gastrointestinal disorders Gastro-intestinal discomfort, anorexia, nausea, vomiting, constipation, diarrhea.
Not known: Renal and urinary disorders Darkening of the stools may occur. Blood amylase, lipase and alkaline phosphatase increased
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: http://www.zuventus.co.in/safety.aspx
4.9 Overdose
Symptoms
Symptoms and signs of abdominal pain, vomiting and diarrhea appear within 60 minutes. Cardiovascular collapse with coma may follow. Some improvement may occur after this phase which, in some patients, is followed by recovery. In others, after about 16 hours, deterioration may occur involving diffuse vascular congestion, pulmonary edema, convulsions, anuria, hypothermia, severe shock, metabolic acidosis, coagulation abnormalities and hypoglycemia.
Management
Vomiting should be induced immediately, followed as soon as possible by parenteral injection of desferrioxamine mesylate, and then gastric lavage. In the meantime, it is helpful to give milk and/or 5% sodium bicarbonate solution by mouth.
Dissolve 2g desferrioxamine mesylate in 2 to 3ml of water for injections and give intramuscularly. A solution of 5g desferrioxamine in 50 to 100ml of fluid may be left in the stomach. If desferrioxamine is not available, leave 300ml of 1 % to 5 % sodium bicarbonate in the stomach. Fluid replacement is essential.
Recovery may be complicated by long-term sequelae such as hepatic necrosis, pyloric stenosis or acute toxic encephalitis which may lead to CNS damage.
Treatment of overdose should be with gastric lavage or induced emesis as quickly as possible to remove unabsorbed zinc. Heavy metal chelation therapy should be considered if plasma zinc levels are markedly elevated (> 10 mg/l).
Pediatric population
Acute overdose of oral iron requires emergency treatment. In young children 200-250mg/kg Ferrous Ascorbate is considered to be extremely dangerous.
5.0 Pharmacological properties
5.1 Mechanism of Action/ Pharmacodynamic properties
Iron absorption occurs predominantly in the duodenum and upper jejunum. Iron is oxidized to the Fe3+ state no matter its original form when taken in orally. Gastric acidity as well as solubilizing agents such as ascorbate prevent precipitation of the normally insoluble Fe3+. Intestinal mucosal cells in the duodenum and upper jejunum absorb the iron. The iron is coupled to transferrin (Tf) in the circulation which delivers it to the cells of the body. A feedback mechanism exists that enhances iron absorption in people who are iron deficient. In contrast, people with iron overload dampen iron absorption. A number of dietary factors influence iron absorption. Ascorbate increase iron uptake in part by acting as weak chelators to help to solubilize the metal in the duodenum. Iron is readily transferred from these compounds into the mucosal lining cells.
Zinc supplementation improves immunity.
Zinc deficiency also has direct effects on the gastrointestinal tract such as impaired intestinal brush border, increased secretion in response to bacterial enterotoxins and perturbations in intestinal permeability. Zinc supplementation improves the transport of water and electrolytes across the intestinal mucosa in experimental zinc deficiency.
Zinc plays a fundamental role in cellular metabolism and is postulated to modulate host resistance to various infections.
5.2 Pharmacokinetic properties
Absorption
Iron is absorbed chiefly in the duodenum and jejunum. Folic Acid is absorbed mainly from the proximal part of the small intestine.
Distribution
The amounts of Folic Acid absorbed from normal diets are rapidly distributed in body tissues.
Biotransformation
Absorption being aided by the acid secretion of the stomach and being more readily affected when the iron is in the ferrous state.
Folic acid rapidly appears in the blood, where it is extensively bound to plasma proteins.
When larger amounts are absorbed, a high proportion is metabolized in the liver to other active forms of folate and a proportion is stored as reduced and methylated folate.
Elimination
Larger amounts of folate are rapidly excreted in the urine and about 4 to 5 micrograms is excreted in the urine daily.
Zinc is absorbed in the small intestine and its absorption kinetics suggest a tendency to saturation at increasing doses. Fractional zinc absorption is negatively correlated with zinc intake. It ranges from 30 to 60% with usual dietary intake (7-15 mg/d) and decreases to 7% with pharmacological doses of 100 mg/d.
In the blood, about 80% of absorbed zinc is distributed to erythrocytes, with most of the remainder being bound to albumin and other plasma proteins. The liver is the main storage for zinc and hepatic zinc levels are increased during maintenance therapy with zinc. The plasma elimination half-life of zinc in healthy subjects is around 1 hour after a dose of 45 mg. The elimination of zinc results primarily from fecal excretion with relatively little from urine and sweat. The fecal excretion is in the greatest part due to the passage of unabsorbed zinc but it is also due to endogenous intestinal secretion.
6.0. Description
This Product (tablet) contains: Ferrous Ascorbate, Folic Acid, Methylcobalamin and Zinc sulphate as active ingredients, for the treatment of iron deficiency anemia.
Ferrous Ascorbate is an iron supplement used to treat or prevent low blood levels of iron (such as those caused by anemia or during pregnancy). Ascorbic acid (vitamin C) improves the absorption of iron from the stomach.
Molecular Formula- C12H14FeO12 Molecular Weight: 406.08g/mol

Folic acid is used to treat anemia caused by folate deficiency. Folic acid is also used as a supplement by women during pregnancy to reduce the risk of neural tube defects (NTDs) in the baby.
Molecular Formula-C19H19N7O6
Molecular Weight- 441.4 g/mol

Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. Zinc is also an essential nutrient element for coral growth as it is an important cofactor for many enzymes.

Molecular Formula-ZnSO4
Molecular Weight- 179.47 g/mol
Methylcobalamin a form of vitamin B12.
Methylcobalamin is equivalent physiologically to vitamin B12, and can be used to prevent or treat pathology arising from a lack of vitamin B12 intake. Methylcobalamin is also used in the treatment of peripheral neuropathy, diabetic neuropathy, and as a preliminary treatment for amyotrophic lateral sclerosis.

Molecular formula: C63H91CoN13O14P
Molecular weight: 1344.405 g·mol−1
8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on pack.
8.3 Packaging information
3 blister strips of 10 tablets each
8.4 Storage and handing instructions
Store at a temperature not exceeding 25°C. Protect from light.
Keep out of reach of children.
9.0 Patient Counselling Information
Patient Counselling Information
- WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor immediately.
- Feronia HP Tablet is given to fulfil your nutritional requirement and to prevent any related diseases.
- Avoid taking antacids 2 hours before or after taking Feronia HP Tablet as they may make it harder for your body to absorb the medicine.
- Let your doctor know if you are taking any other medications like antihypertensive, antibiotics, or medicines for heart disease or bone disorders.
12.0 Date of revision
Sept 2024
Read this entire leaflet carefully before you start taking this medicine because it contains important information for you
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
1. What Feronia HP Tablets is and what it is used for?
Feronia HP film-coated Tablets (referred to as Feronia HP in this leaflet) contains Ferrous Ascorbate 100 mg, Folic Acid 1.5 mg, Methylcobalamin 1.5 mg, Zinc Sulphate 22.5mg. These work together in the medicine.
Feronia HP belongs to a group of medicines called haematinics and vitamin, mineral supplement.
Feronia HP works as a supplement. It provides the body with more iron and folic acid. These are important substances that your body needs to form red blood cells. If you do not have the right amount of these substances, it is possible that you may develop anemia.
Feronia HP is used to prevent and treat low levels of iron and folic acid in the blood (such as those caused by anemia or during pregnancy). Ascorbic acid (vitamin C) improves the absorption of iron from the stomach. Zinc supplementation improves immunity. Methylcobalamin is a form of Vitamin B12, important for the brain and nerves and for the production of red blood cells.
2. What you need to know before you take Feronia HP Tablets?
Do not take Feronia HP:
- if you are allergic to Ferrous Ascorbate and Folic Acid or any of the other ingredients of this medicine
- if you are breast-feeding or trying to become pregnant
- if you suffer from a blood disorder
- if you have had or are having repeated blood transfusions
- if you have a stomach ulcer or other digestive conditions such as regional enteritis or ulcerative colitis
- if you are suffering from anemia that is not due to a lack of iron
If any of the above applies to you, talk to your doctor or pharmacist.
Warnings and precautions
Talk to your doctor before taking Feronia HP
- if you have been or you are being treated for a stomach ulcer
- if you have had or you have a folate dependent tumour
- if you have had all or part of your stomach removed
Feronia HP contains iron. Keep out of reach and sight of children, as overdose may be fatal.
Children
There is no relevant use of Feronia HP in children.
Other medicines and Feronia HP
Tell your doctor if you are taking any other medicines.
- Antibiotics e.g. fluoroquinolones, cotrimoxazole, chloramphenicol, sulphonamides, tetracyclines, neomycin (used for infections)
- Anticonvulsant medicines (used for epilepsy)
- Antacids
- Penicillamine (used for rheumatoid arthritis)
- Sulfasalazine (used for rheumatoid arthritis and bowel disease, e.g. Crohn’s disease)
- Cholestyramine (used for reducing blood cholesterol or control diarrhea)
- Levodopa or Carbidopa (used for Parkinson’s disease)
- Thyroxine (used for thyroid disease)
- Bisphosphonates (used for bone disease)
- Aminopterin and Methotrexate (used for certain cancers)
- Pyrimethamine (used for malaria)
- Trientine (used for Wilson’s disease)
- Methyldopa (used for high blood pressure)
- Any other medicine, including medicines obtained without a prescription
Feronia HP with food and drink
If you drink tea, coffee or milk or eat eggs at the same time as taking Feronia HP, your body may absorb less of the iron and zinc supplement, which may reduce the effect of this medicine.
Pregnancy and breast-feeding
If you are pregnant or think you may be pregnant, ask your doctor for advice before taking this medicine.
Driving and using machines.
There are no known effects on driving or using machines.
3. How to take Feronia HP Tablets?
Always take Feronia HP Tablets exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The recommended dose for adults
- The usual dose is one tablet each day to be taken by mouth.
Method of administration: For oral administration only.
Swallow the tablet whole with a glass of water.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek immediate medical help if you have an allergic reaction.
This includes any of the following symptoms:
- Difficulties in breathing
- Swelling of your eyelids, face or lips
- Rash or itching
Not known: frequency cannot be estimated from the available data
- Upset stomach
- Anorexia (e.g. loss of appetite)
- Sickness
- Constipation
- Diarrhea
- Darkening of stools
Reporting of side effects: If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.You can also report side effects directly: Website: www.zuventus.com in and click the tab “Safety Reporting” located on the top end of the home page.
Website link: https://www.zuventus.co.in/drug-safety-reporting
5. How should I store Feronia HP Tablets?
- Keep this medicine out of the sight and reach of children. An overdose can be fatal.
- Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of that month.
- Store below 25°C. Keep the blister in the outer carton in order to protect from light.
- Do not throw away any medicines via wastewater or household waste.
- Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
- Ferrous Ascorbate 100 mg
- Folic Acid 1.5 mg
- Methylcobalamin 1.5 mg
- Zinc Sulphate 22.5mg
Pack size:
3 blister strips of 10 tablets each
For More Information About This Product
Feronia D3 Tablets
1.0 Generic Name
Ferrous Ascorbate, Folic Acid, Cyanocobalamin, Pyridoxine Hydrochloride & Cholecalciferol Tablets
2.0 Qualitative and Quantitative Composition
Each film coated tablet contains:
Ferrous Ascorbate IP
equivalent to Elemental Iron 100 mg
Folic Acid IP 1.5 mg
Cyanocobalamin IP 7.5 mcg
(Vitamin B12)
Pyridoxine Hydrochloride IP 1.5 mg
(Vitamin B6)
Cholecalciferol IP 1000 IU (Vitamin D3) (As stabilized form) Excipients q.s. Colours: Lake of Sunset Yellow,
Lake of Erythrosine & Titanium Dioxide IP
3.0 Dosage Form and Strength
Film coated tablet
Ferrous ascorbate (100mg), Folic Acid (1.5mg), Cyanocobalamin (7.5 mcg), Pyridoxine Hydrochloride (1.5 mg), Cholecalciferol IP (1000 IU)
4.0 Clinical particulars
4.1 Therapeutic indications
Treatment and prevention of anemia with multivitamin deficiency
4.2 Posology and method of administration
Adults
One tablet to be taken daily orally
Pediatric Population
There is no relevant use of Feronia-D3 Tablets in the pediatric population.
4.3 Contraindications
- Hypersensitivity to the active substances or any of the excipients.
- Paroxysmal nocturnal hemoglobinuria, hemosiderosis, haemochromatosis, active peptic ulcer, repeated blood transfusion, regional enteritis and ulcerative colitis.
- Hypercalcaemia and/or hypercalciuria
- Nephrolithiasis (Renal calculi)
- Hypervitaminosis
- Severe renal impairment
4.4 Special warnings and precautions for use
- CAUTION: Tablets are hygroscopic in nature. Exposure to humid conditions may soften the tablets affecting their appearance and shape. Cutting of strip to be avoided while dispensing /usage.
- Important warning: Contains Iron. Keep out of reach and sight of children, as overdose may be fatal.
- Some post-gastrectomy patients show poor absorption of iron. Care is needed when treating iron deficiency anemia in patients with treated or controlled peptic ulceration. Caution should be exercised when administering folic acid to patients who may have folate dependent tumors.
- Since anemia due to combined iron and vitamin B12 or folate deficiencies may be microcytic in type, patients with microcytic anemia resistant to therapy with iron alone should be screened for vitamin B12 or folate deficiency.
- Cholecalciferol should be used with caution in patients with impairment of renal function and the effect on calcium and phosphate levels should be monitored. The risk of soft tissue calcification should be taken into account. In patients with severe renal insufficiency, vitamin D in the form of Cholecalciferol is not metabolised normally and other forms of vitamin D should be used. Cholecalciferol should not be taken by patients with a tendency to form calcium-containing renal calculi.
- Caution is required in patients receiving treatment for cardiovascular disease
Pediatric population
Feronia D3 Tablets should be kept out of the reach of children.
4.5 Interaction with other medicinal products and other forms of interaction
- Iron reduces the absorption of penicillamine. Iron compounds impair the bioavailability of fluoroquinolones, levodopa, carbidopa, thyroxine and bisphosphonates.
- Absorption of both iron and antibiotic may be reduced if Feronia D3 is given with tetracycline.
- Absorption of both iron and zinc are reduced if taken concomitantly. Concurrent administration of antacids may reduce absorption of iron. Co- trimoxazole, chloramphenicol, sulphasalazine, aminopterin, methotrexate, pyrimethamine or sulphonamides may interfere with folate metabolism. Serum levels of anticonvulsant drugs may be reduced by administration of folate.
- Oral chloramphenicol delays plasma iron clearance, incorporation of iron into red blood cells and interferes with erythropoiesis.
- Patients treated with cardiac glycosides may be susceptible to high calcium levels and should have ECG parameters and calcium levels monitored. It is recommended to reduce the dose or interrupt treatment if the calcium content in the urine exceeds 7.5 mmol/24 hours (300 mg/24 hours).
- Simultaneous administration of benzothiadiazine derivatives (thiazide diuretics) increases the risk of hypercalcaemia because they decrease the calcium excretion in the urine. The calcium levels in plasma and urine should therefore be monitored for patients undergoing long-term treatment.
- If Cholecalciferol is combined with metabolites or analogues of vitamin D careful monitoring of serum calcium levels is recommended.
- Some inhibition of iron absorption may occur if it is taken with cholestyramine, trientine, tea, eggs or milk.
- Administration of oral iron may increase blood pressure in patients receiving methyldopa.
- Coffee may be a factor in reducing iron bioavailability. Neomycin may alter the absorption of iron.
- Anti-convulsants e.g. phenytoin, phenobarbital, primidone may diminish the effect of Cholecalciferol due to hepatic enzyme induction.
- Rifampicin may reduce the effectiveness of Cholecalciferol due to hepatic enzyme induction.
- Isoniazid may reduce the effectiveness of Colecalciferol due to inhibition of the metabolic activation of Colecalciferol.
- Drugs leading to fat malabsorption, e.g. orlistat, liquid paraffin, cholestyramine, may impair the absorption of Cholecalciferol.
- The cytotoxic agent actinomycin and imidazole antifungal agents interfere with vitamin D activity.
- Concomitant use of glucocorticoids can decrease the effect of vitamin D.
4.6 Fertility, pregnancy and lactation
Pregnancy
The use of Feronia D3 Tablets may be considered during pregnancy, if necessary.
The development of anemia despite prophylaxis with Feronia D3 Tablets calls for investigation. During pregnancy women should follow the advice of their medical practitioner as their requirements may vary depending on the severity of their disease and their response to treatment.
Breast-feeding
It is unknown whether Ferrous Ascorbate and Folic Acid / metabolites are excreted in human milk.
A risk to the newborns/infants cannot be excluded.
Cholecalciferol and its metabolites are excreted in breast milk. Overdose in infants induced by nursing mothers has not been observed. However, when prescribing additional vitamin D to a breast-fed child the practitioner should consider the dose of any additional vitamin D given to the mother.
Fertility
No fertility data is available.
4.7 Effects on ability to drive and use machines
Feronia D3 Tablets has no influence on the ability to drive and use machines. Cholecalciferol has no known side effects that are likely to affect the ability to drive and use or operate machines.
4.8 Undesirable effects
Very rare (<1/10,000)
Very rare (<1/10,000): Rarely allergic reactions may occur. Not known (cannot be estimated from the available data) Not known: Gastrointestinal disorders Gastro-intestinal discomfort, anorexia, nausea, vomiting, constipation, diarrhea. Not known: Renal and urinary disorders Darkening of the stools may occur. Rarely Hypercalcemia, Hypercalciuria
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: Website: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this medicine.
4.9 Overdose
Symptoms
Symptoms and signs of abdominal pain, vomiting and diarrhea appear within 60 minutes. Cardiovascular collapse with coma may follow. Some improvement may occur after this phase which, in some patients, is followed by recovery. In others, after about 16 hours, deterioration may occur involving diffuse vascular congestion, pulmonary edema, convulsions, anuria, hypothermia, severe shock, metabolic acidosis, coagulation abnormalities and hypoglycemia.
Acute or chronic overdose of Cholecalciferol can cause hypercalcaemia, an increase in the serum and urinary concentrations of calcium. The symptoms of hypercalcaemia are not very specific and consist of nausea, vomiting, diarrhoea often in the early stages and later constipation, anorexia, fatigue, headache, muscle and joint pain, muscle weakness, polydipsia, polyuria formation of renal calculi, nephrocalcinosis, kidney failure, calcification of soft tissues, changes in ECG measurements, arrhythmias and pancreatitis. In rare and isolated cases there are reports that hypercalcaemia is fatal.
Management
Vomiting should be induced immediately, followed as soon as possible by parenteral injection of desferrioxamine mesylate, and then gastric lavage. In the meantime, it is helpful to give milk and/or 5% sodium bicarbonate solution by mouth.
Dissolve 2g desferrioxamine mesylate in 2 to 3ml of water for injections and give intramuscularly. A solution of 5g desferrioxamine in 50 to 100ml of fluid may be left in the stomach. If desferrioxamine is not available, leave 300ml of 1 % to 5 % sodium bicarbonate in the stomach. Fluid replacement is essential.
Recovery may be complicated by long-term sequelae such as hepatic necrosis, pyloric stenosis or acute toxic encephalitis which may lead to CNS damage.
A normalisation of hypercalcaemia due to vitamin D intoxication lasts several weeks. The recommendation for the treatment of hypercalcaemia is the avoidance of any further administration of vitamin D, including supplements, dietary intakes and the avoidance of sunlight. A low calcium or calcium-free diet can also be considered.
Rehydration and the treatment with diuretics e.g. furosemide to ensure adequate diuresis should be considered. Additional treatment with calcitonin or corticosteroids can also be considered.
Phosphate infusions should not be administered to lower hypercalcaemia of hypervitaminosis D because of the dangers of metastatic calcification.
Pediatric population
Acute overdose of oral iron requires emergency treatment. In young children 200-250mg/kg Ferrous Ascorbate is considered to be extremely dangerous.
5.0 Pharmacological properties
5.1 Mechanism of Action/ Pharmacodynamic properties
Iron absorption occurs predominantly in the duodenum and upper jejunum. Iron is oxidized to the Fe3+ state no matter its original form when taken in orally. Gastric acidity as well as solubilizing agents such as ascorbate prevent precipitation of the normally insoluble Fe3+. Intestinal mucosal cells in the duodenum and upper jejunum absorb the iron. The iron is coupled to transferrin (Tf) in the circulation which delivers it to the cells of the body. A feedback mechanism exists that enhances iron absorption in people who are iron deficient. In contrast, people with iron overload dampen iron absorption. A number of dietary factors influence iron absorption. Ascorbate increase iron uptake in part by acting as weak chelators to help to solubilize the metal in the duodenum. Iron is readily transferred from these compounds into the mucosal lining cells.
Cholecalciferol is produced within the skin under the influence of UV radiation including sunlight. In its biologically active form, Cholecalciferol stimulates intestinal calcium absorption, incorporation of calcium into the osteoid, and release of calcium from bone tissue. In the small intestine it promotes rapid and delayed calcium uptake. The passive and active transport of phosphate is also stimulated. In the kidney, it inhibits the excretion of calcium and phosphate by promoting tubular resorption. The production of parathyroid hormone (PTH) in the parathyroids is inhibited directly by the biologically active form of Cholecalciferol. PTH secretion is inhibited additionally by the increased calcium uptake in the small intestine under the influence of biologically active Cholecalciferol.
Elimination: Cholecalciferol and other forms of vitamin D are excreted in feces and urine.
- Iron participates in the second activation step of vitamin D, necessary to make this hormone functional.
- This conversion is done by a renal 25-hydroxyvitamin D 1α-hydroxylase (1α-OHase) enzyme which comprises a CYP-450, a ferredoxin, and a ferredoxin reductase.
- Therefore, less available iron could compromise production of the active form of vitamin D.
5.2 Pharmacokinetic properties
Folic Acid is absorbed mainly from the proximal part of the small intestine.
Distribution
The amounts of Folic Acid absorbed from normal diets are rapidly distributed in body tissues.
Biotransformation
Absorption being aided by the acid secretion of the stomach and being more readily affected when the iron is in the ferrous state.
Folic acid rapidly appears in the blood, where it is extensively bound to plasma proteins.
When larger amounts are absorbed, a high proportion is metabolized in the liver to other active forms of folate and a proportion is stored as reduced and methylated folate.
Elimination
Larger amounts of folate are rapidly excreted in the urine and about 4 to 5 micrograms is excreted in the urine daily.
The pharmacokinetics of Cholecalciferol have been widely studied and are well-known. Cholecalciferol from nutritional sources is almost completely absorbed from within the gastro-intestinal tract in the presence of dietary lipids and bile acids. Cholecalciferol is stored in fat cells and its biological half-life is approximately 50 days.
Cholecalciferol is metabolised by microsomal hydroxylase to form 25-hydroxycolecalciferol (25(OH)D3, calcidiol), the primary storage form of vitamin D3. 25(OH)D3 undergoes a secondary hydroxylation within the kidney to form the predominant active metabolite 1,25-hydroxycolecalciferol (1,25(OH)2D3, calcitriol). The metabolites circulate in the blood bound to a specific α -globin.
After a single oral dose of Cholecalciferol, the maximum serum concentrations of the primary storage form are reached after approximately 7 days. 25(OH)D3 is then slowly eliminated with an apparent half-life in serum of about 50 days. Cholecalciferol and its metabolites are excreted mainly in the bile and feces.
After high doses of Cholecalciferol, serum concentrations of 25(OH)D3 may be increased for months. Overdose-induced hypercalcemia may persist for weeks
6.0 Description
This Product (tablet) contains: Ferrous Ascorbate, Folic Acid Cyanocobalamin, Pyridoxine and Cholecalciferol as active ingredients, for the treatment and prevention of Anemia with multivitamin deficiency.
Ferrous Ascorbate is an iron supplement used to treat or prevent low blood levels of iron (such as those caused by anemia or during pregnancy). Ascorbic acid (vitamin C) improves the absorption of iron from the stomach.

Vitamin D promotes calcium absorption in the gut and maintains adequate serum calcium and phosphate concentrations to enable normal bone mineralization and to prevent hypocalcemic tetany.

Folic acid is used to treat anemia caused by folate deficiency. Folic acid is also used as a supplement by women during pregnancy to reduce the risk of neural tube defects (NTDs) in the baby.
Molecular Formula-C19H19N7O6
Molecular Weight- 441.4 g/mol

8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on pack.
8.3 Packaging information
3 Blister strips of 10 tablets each
8.4 Storage and handing instructions
Store in a cool & dry place. Protect from moisture. Protect from light. Keep out of reach of children.
9.0 Patient Counselling Information
- WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor immediately.
- Feronia -D3 Tablet is given to fulfill your nutritional requirement and to prevent any related diseases.
- Avoid taking antacids 2 hours before or after taking Feronia-D3 Tablet as they may make it harder for your body to absorb the medicine.
- Let your doctor know if you are taking any other medications like antihypertensive, antibiotics, or medicines for heart disease or bone disorders.
12.0 Date of revision
25th Sept 2024
Read this entire leaflet carefully before you start taking this medicine because it contains important information for you
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
1. What Feronia D3 Tablets is and what it is used for?
Feronia D3 film-coated Tablets (referred to as Feronia D3 in this leaflet) contains active substances Ferrous Ascorbate 100mg (an iron supplement) and Folic Acid 1.5mg, Cyanocobalamin (Vitamin B12) IP-7.5 mcg, Pyridoxine Hydrochloride (Vitamin B6) IP-1.5 mg, Cholecalciferol (Vitamin D3) IP-1000 IU. These work together in the medicine.
Feronia D3 belongs to a group of medicines called haematinics and multivitamins supplement.
Feronia D3 works as a supplement. It provides the body with more iron and folic acid, vitamin D3, B12, B6. These are important substances that your body needs to form red blood cells, bone mineralisation. If you do not have the right amount of these substances, it is possible that you may develop anemia, osteoporosis.
Feronia D3 is used to prevent and treat low levels of iron and folic acid, vitamin D, B12, B6 in the blood (such as those caused by anemia or during pregnancy). Ascorbic acid (vitamin C) improves the absorption of iron from the stomach.
2. What you need to know before you take Feronia D3 Tablets?
Do not take Feronia D3:
- if you are allergic to any of the other ingredients of this medicine
- if you are breast-feeding or trying to become pregnant
- if you have been told you suffer from Vitamin B12 deficiency
- if you suffer from a blood disorder
- if you have had or are having repeated blood transfusions
- if you have a stomach ulcer or other digestive conditions such as regional enteritis or ulcerative
colitis
- if you are suffering from anemia that is not due to a lack of iron
- If any of the above applies to you, talk to your doctor or pharmacist.
Warnings and precautions
Talk to your doctor before taking Feronia D3
- if you have been or you are being treated for a stomach ulcer
- if you have had or you have a folate dependent tumour
- if you have had all or part of your stomach removed
Feronia D3 contains iron. Keep out of reach and sight of children, as overdose may be fatal.
Children
There is no relevant use of Feronia D3 in children.
Other medicines and Feronia D3
Tell your doctor if you are taking any other medicines.
- Antibiotics e.g. fluoroquinolones, cotrimoxazole, chloramphenicol, sulphonamides, tetracyclines, neomycin (used for infections)
- Anticonvulsant medicines (used for epilepsy)
- Antacids
- Penicillamine (used for rheumatoid arthritis)
- Sulfasalazine (used for rheumatoid arthritis and bowel disease, e.g. Crohn’s disease)
- Cholestyramine (used for reducing blood cholesterol or control diarrhea)
- Levodopa or Carbidopa (used for Parkinson’s disease)
- Thyroxine (used for thyroid disease)
- Bisphosphonates (used for bone disease)
- Aminopterin and Methotrexate (used for certain cancers)
- Pyrimethamine (used for malaria)
- Trientine (used for Wilson’s disease)
- Methyldopa (used for high blood pressure)
- Zinc
- Any other medicine, including medicines obtained without a prescription
Feronia D3 with food and drink
If you drink tea, coffee or milk or eat eggs at the same time as taking Feronia D3 your body may absorb less of the iron supplement, which may reduce the effect of this medicine.
Pregnancy and breast-feeding
If you are pregnant or think you may be pregnant, ask your doctor for advice before taking this medicine.
Driving and using machines.
There are no known effects on driving or using machines.
3. How to take Feronia D3 Tablets?
Always take Feronia D3 Tablets exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The recommended dose for adults
- The usual dose is one tablet each day to be taken by mouth.
Method of administration: For oral administration only.
Swallow the tablet whole with a glass of water.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Seek immediate medical help if you have an allergic reaction.
This includes any of the following symptoms:
- Difficulties in breathing
- Swelling of your eyelids, face or lips
- Rash or itching Not known: frequency cannot be estimated from the available data
- Upset stomach
- Anorexia (e.g. loss of appetite)
- Sickness
- Constipation
- Diarrhea
- Darkening of stools
Reporting of side effects: If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.You can also report side effects directly: Website: www.zuventus.com in and click the tab “Safety Reporting” located on the top end of the home page.
Website link: https://www.zuventus.com/drug-safety-reporting
By reporting side effects, you can help provide more information on the safety of this product.
5. How should I store Feronia D3 Tablets?
- Keep this medicine out of the sight and reach of children. An overdose can be fatal.
- Do not use this medicine after the expiry date which is stated on the carton and blister after EXP. The expiry date refers to the last day of that month.
- Store below 25°C. Keep the blister in the outer carton in order to protect from light.
- Do not throw away any medicines via wastewater or household waste.
- Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
- The active substances are Ferrous Ascorbate, Folic Acid, Cyanocobalamin, Pyridoxine and Cholecalciferol
- Each tablet contains Ferrous Ascorbate 100mg, Folic Acid 1.5mg, Cyanocobalamin IP-7.5 mcg, Pyridoxine Hydrochloride IP-1.5 mg, Cholecalciferol IP-1000 IU Pack size: 3 Blister strips of 10 tablets each
For More Information About This Product
Efnocar 40 Tablet
1.0 Generic Name
Efonidipine Hydrochloride Ethanolate Tablets 10 mg / 20 mg / 40 mg
2.0 Qualitative and quantitative composition
EFNOCAR-10
Each film coated tablet contains:
Efonidipine Hydrochloride Ethanolate 10 mg
Excipients q.s.
Colour: Titanium Dioxide IP
EFNOCAR-20
Each film coated tablet contains:
Efonidipine Hydrochloride Ethanolate 20 mg
Excipients q.s.
Colour: Titanium Dioxide IP
EFNOCAR-40
Each film coated tablet contains:
Efonidipine Hydrochloride Ethanolate 40 mg
Excipients q.s.
Colour: Titanium Dioxide IP
3.0 Dosage form and strength
Film coated tablet
10/ 20/40 mg
4.0 Clinical particulars
4.1 Therapeutic Indication
Efonidipine is indicated for the management of
- Essential hypertension and renal parenchymal hypertension
- Angina
4.2 Posology and method of administration
Adults
- Essential hypertension and renal parenchymal hypertension: 20-40 mg orally once daily. A dose of up to 80 mg/day has been reported to be safe and effective in clinical trials.
- Angina: 40 mg/day.
Elderly
Efonidipine should be started with a low dose (20mg/day) and the patient's condition should be monitored. The dose should be halved in case the patient is intolerant to the higher dose.
In Children
Efonidipine is not recommended in infants and children as safety is not established in this group of patients.
4.3 Contraindications
- In patients with known hypersensitivity to Efonidipine or any other component of the formulation
- In pregnant women
4.4 Special warnings and precautions for use
- Should be administered with caution in patients with hepatic impairment.
- Should be administered with caution in patients with low BP and/or sinus node dysfunction.
- The drug should be withdrawn gradually to prevent rebound hypertension or worsening of angina.
- Administration of the drug may cause hypotension. Under such circumstances appropriate measures should be taken to either reduce the dose or withdraw the drug.
- Should not be taken with grapefruit juice as there may be excessive lowering of blood pressure.
- Dizziness may occur while taking antihypertensive agents. Hence, working on aerial platform, working with dangerous machinery and/or driving should be avoided.
- To be sold by retail on the prescription of Cardiologist / Nephrologist / Endocrinologist and Specialist in General / Internal medicine / Critical Care medicine only.
4.5 Drugs interactions
Concomitant administration of other antihypertensive agent/s may enhance the antihypertensive effect of efonidipine.
- Administration of calcium channel blockers (CCBs) with Cimetidine may cause elevated levels of CCBs.
- Increased levels of CCBs observed when taken concomitantly with grapefruit juice which may result in excessive lowering of blood pressure.
- Efonidipine when taken along with Tacrolimus may cause increased blood levels of Tacrolimus.
4.6 Use in special populations
Pregnancy
Efonidipine should not be administered in pregnant women.
Nursing Mothers
Efonidipine should not be administered in lactating women.
4.7 Effects on ability to drive and use machines
If patients taking Efonidipine suffer from dizziness, headache, fatigue or nausea the ability to react may be impaired. Caution is recommended especially at the start of treatment.
4.8 Undesirable effects
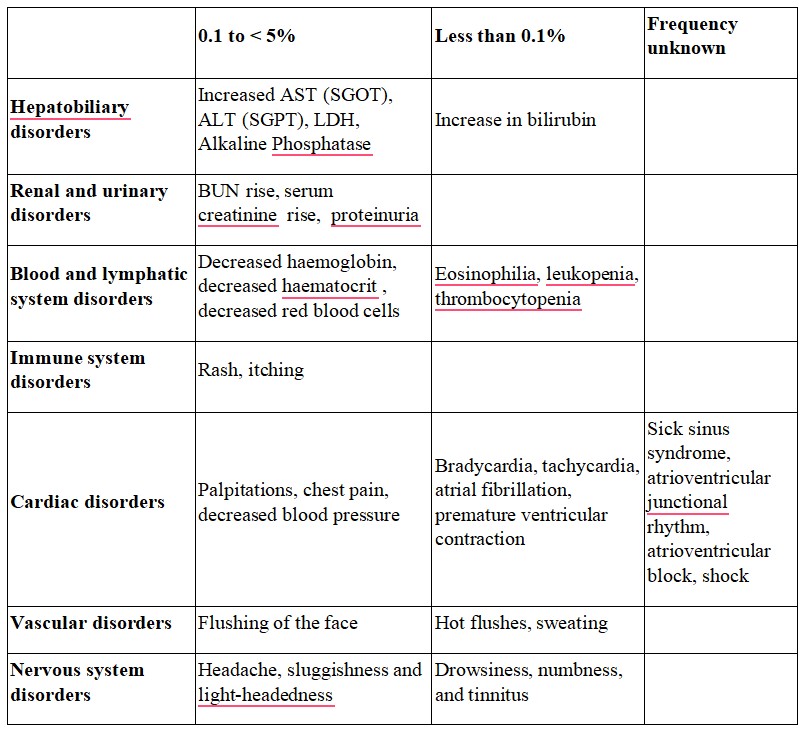
The common side effects are hot flushes, palpitations, facial flushing and headache. In addition, elevation in serum total cholesterol, ALT (SGPT), AST (SGOT) and BUN may occur.
Tabulated list of adverse reactions
Reporting of suspected adverse reactions
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
4.9 Overdose
In humans, experience with intentional overdose is limited. The stomach should be emptied by aspiration and gastric lavage if the patient reports immediately or up to two hours after overdosage.
Clinically significant hypotension due to Efonidipine overdosage calls for active cardiovascular support including frequent monitoring of cardiac and respiratory function, elevation of extremities, and attention to circulating fluid volume and urine output. A vasoconstrictor may be helpful in restoring vascular tone and blood pressure, provided that there is no contraindication to its use. Intravenous calcium gluconate may be beneficial in reversing the effects of calcium channel blockade.
5.0 Pharmacological properties
5.1 Mechanism of Action
Efonidipine, a new generation dihydropyridine (DHP) calcium channel blocker, inhibits both L-type and T-type calcium channels.
5.2 Pharmacodynamic properties
- Efonidipine exhibits antihypertensive effect through vasodilatation by blocking L-type and T-type calcium channels.
- Efonidipine has a negative chronotropic effect. Working on sino atrial node cells by inhibiting T-type calcium channel activation, Efonidipine prolongs the late phase-4 depolarization of the sino atrial node action potential and suppresses an elevated HR. The negative chronotropic effect of Efonidipine decreases heart rate, myocardial oxygen demand and increases coronary blood flow.
- Efonidipine increases coronary blood flow by blocking L & T-type calcium channels and attenuates myocardial ischaemia.
- By reducing synthesis and secretion of aldosterone, Efonidipine prevents hypertrophy and remodeling of cardiac myocytes.
- Efonidipine increases glomerular filtration rate without increasing intra-glomerular pressure and filtration fraction. This prevents hypertension induced renal damage.
- Efonidipine prevents Rho-kinase and NF-kB induced renal parenchymal fibrosis and provides long term renal protection.
- Efonidipine suppresses renin secretion from the Juxta Glomerular apparatus in the kidneys. •Efonidipine enhances sodium excretion from the kidneys by suppressing aldosterone synthesis and secretion from the adrenal glands. Aldosterone induced renal parenchymal fibrosis is suppressed by Efonidipine. •Efonidipine prevents NF-kB induced hypertrophy and inflammation in the renal vasculature and protects the kidneys.
- Efonidipine protects against endothelial dysfunction due to its anti-oxidant activity and by restoring nitric oxide bioavailability.
- Efonidipine has anti-atherogenic activity and protects the blood vessels from atherosclerosis.
- Efonidipine lowers blood pressure in cerebral resistance vessels and prevents hypertension induced brain damage.
5.3 Pharmacokinetic properties
Absorption
Peak plasma concentration is achieved in about 1.5 to 3.67 hours after administration. The bioavailability of Efonidipine is ~25% and half-life is approximately 4 hours.
Efonidipine 40mg when administered to healthy volunteers under fasting conditions shows the following pharmacokinetic profile:
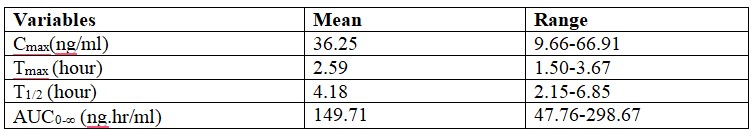
The dissociation constant of Efonidipine from dihydropyridine receptors is very low (0.0042/min/nM), signifying very slow dissociation from the receptors. This explains the long duration of action of Efonidipine.
Metabolism
Efonidipine is primarily metabolized in the liver. The important metabolites are N-dephenylated Efonidipine (DPH), deaminated Efonidipine (AL) and N-debenzylated Efonidipine (DBZ). DBZ and DPH exhibit activity as calcium antagonists. The vasodilating properties of DBZ and DPH were about two-thirds and one-third respectively than that of the parent compound. Results suggest that the majority of the pharmacological effect after oral dosing of Efonidipine hydrochloride in man is due to unchanged compound and its metabolites make a small contribution to the pharmacological effect.
Elimination
Biliary route is the main pathway of excretion. No significant amount of unchanged drug is excreted in urine. In the urine collected for 24 h after an oral dosing, 1.1% of the dose was excreted as deaminated Efonidipine, and 0.5% as a pyridine analogue of deaminated Efonidipine.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
The acute toxicology of efonidipine was studied in male and female mice, rats, and dogs following oral or intravenous administration. The intravenous LD, values in male mice and rats were 77 and 51 mg/kg, respectively. The oral LD, values for efonidipine in mice, rats, and dogs were higher than 1,500 mag. The signs of toxicity observed in mice and rats were depression of spontaneous movement, hypothermia, sedation, piloerection, lying, gasping, and clonic convulsion. The dogs developed mild diarrhea, soft stools, or a temporary decrease in food consumption.
A 13-week oral subacute toxicity study of efonidipine was performed in male or female rats and dogs. Increases in water consumption and urine volume were observed in male and female rats receiving efonidipine at 300 mg/kg/day. Urinalysis revealed a high concentration of Na+ and C1- in the rats receiving 100 or 300 mg/kg/day. Organ weight measurement revealed an increase in liver and heart weight in male animals receiving 10 mg/kg/day or more and in female animals receiving 30 mg/kg/day or more.
Thus, in this study, the noneffective dose level was 3 mg/kg/day in rats. The effects observed at 300 or 100 mg/kg/day in groups of male or female dogs included reversible hyperemia of the sclera. Pathological examination revealed a dose-induced increase in the absolute and relative heart weight in female dogs treated with the drug at 100 mg/kg/day or more. Thus, the maximum noneffective dose of efonidipine in dogs of either sex was estimated to be around 30 mg/kg/day.
An oral chronic toxicity study of efonidipine was carried out in rats and dogs. Efonidipine was administered to rats at a daily dose of 1.5, 7, or 30 mg/kg for one year.
Organ weight measurement revealed an increase or a tendency of increase in heart weight in male or female rats receiving 30 mg/kg/day. A decreased thyroid weight in the males and an elevated liver weight in the females were found in the 30 mg/kg/day group.
Therefore, the noneffective estimated oral dose of efonidipine in rats was 7 mg/kg/day. Efonidipine in a gelatin capsule containing 2.0, 6.5, or 20 mg/kg/day was administered to dogs once daily for one year. The estimated effective dose was ca 20 mg/kg/day and the noneffective dose was ca 6.5 mg/kg/day
7.0 Description
Efonidipine is a novel dihydropyridine calcium channel blocker having anti-hypertensive and anti-anginal properties.
Chemical name: (±) -2 - [benzyl (phenyl) amino] ethyl 1, 4-dihydro-2 ,6-dimethyl- 5-(5,5-dimethyl-2-oxo-1 ,3,2-dioxaphosphorinan-2-yl) -4 - (3-nitrophenyl) -3-pyridinecarboxylate hydrochloride ethanol.
Molecular formula: C34H38 N3O7P • HCl •C2H6O
Molecular mass: 714.18 g/mol

8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on pack
8.3 Packaging information
10 Blister strips of 10 tablets each
8.4 Storage and handing instructions
- Store below 25°C. Protected from light & moisture.
- Keep out of reach of children.
9.0 Patient Counselling Information
Efnocar is a type of medicine known as a calcium channel blocker (CCB). It is used to treat high blood pressure (hypertension) and a type of chest pain called angina. It can be used by itself or with other medicines to treat these conditions. Do not use Efnocar if you are allergic to efonidipine (the active ingredient in Efnocar), or to the inactive ingredients.
Tell your doctor about any prescription and non-prescription medicines you are taking, including natural or herbal remedies. Tell your doctor if you:
- ever had heart disease
- ever had liver problems
- are pregnant, or plan to become pregnant.
- are breast-feeding. Do not breast-feed while taking Efnocar.
You can stop breast-feeding or take a different medicine.
It may be easier to take your dose if you do it at the same time every day, such as with breakfast or dinner, or at bedtime.
While you are taking Efnocar do not stop taking your other prescription medicines, including any other blood pressure medicines, without talking to your doctor.
12.0 Date of revision
09/10/2024
About Leaflet
The name of your medicine is EFNOCAR 10 mg / 20 mg / 40 mg Tablets. We refer to them as EFNOCAR Tablets or EFNOCAR throughout this leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any more questions, please ask your doctor or your pharmacist.
This medicine has been prescribed for you personally and you should not pass it on to anyone else. It may harm them, even if their symptoms are the same as yours.
If any of the side effects get serious, or if you notice any side effects that are not listed in the leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What EFNOCAR Tablets are and what they are used for
2. What you need to know before you take EFNOCAR Tablets
3. How to take EFNOCAR Tablets
4. Possible side effects
5. How to store EFNOCAR Tablets
6. Contents of the pack and other information
1. What Efnocar Tablets Are and What They Are Used for
EFNOCAR Tablets contain the active substance efonidipine which belongs to a group of medicines called calcium antagonists.
EFNOCAR Tablets may be used to treat:
- High blood pressure (hypertension)
- hypertension caused by kidney disease
- A certain type of chest pain called angina
In patients with high blood pressure, these medicines work by relaxing blood vessels, so that blood passes through them more easily. In patients with angina, EFNOCAR works by improving blood supply to the heart muscle which then receives more oxygen and as a result chest pain is prevented. EFNOCAR Tablets do not immediately relieve chest pain caused by angina.
2. What You Need to Know Before You Take Efnocar Tablets
Do not take EFNOCAR Tablets if you:
- Have ever had an allergic reaction to efonidipine or any of the ingredients in the tablet. An allergic reaction may include a rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue;
- If you are pregnant
Take special care with EFNOCAR Tablets
You should inform you doctor if you have or have had any of the following conditions:
Liver disease;
Recent heart attack;
Heart failure;
Severe increase in blood pressure (Hypertensive crisis).
Use in children and adolescents
EFNOCAR is not recommended in infants and children as safety is not established in this group of patients.
For more information, talk to your doctor.
Taking other medicines and EFNOCAR
Please tell your doctor or pharmacist if you are taking or have recently taken other medicines, including medicines obtained without a prescription.
EFNOCAR may affect or be affected by other medicines, such as:
- Other antihypertensive agent/s (BP lowering drugs)
- Cimetidine (stomach acid reducer)
- Tacrolimus (a drug to suppress immunity)
EFNOCAR may lower your blood pressure even more if you are already taking other medicines to treat your high blood pressure.
If you see another doctor or go into hospital for any reason, tell them that you are taking EFNOCAR Tablets.
Taking EFNOCAR Tablets with food and drink
You should not drink grapefruit juice or eat grapefruit while taking this medicine. Grapefruit and grapefruit juice can lead to an increase in the blood levels of efonidipine, which can cause an unpredictable increase in its blood pressure lowering effect.
Pregnancy
The safety of efonidipine in human pregnancy has not been established. Efonidipine should not be administered in pregnant women.
Breast-feeding
It is not known whether efonidipine is passed into breast milk. Efonidipine should not be administered in breast-feeding women.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Dizziness may occur while taking antihypertensive agents. Hence, working on aerial platform, working with dangerous machinery and/or driving should be avoided.
3. How to Take Efnocar Tablets
Swallow these tablets with a glass of water at the same time each day. You can take the tablets after meals.
Follow your doctor’s instructions. Check the pharmacy label to see how many tablets to take and how often to take them. If you are still not sure, ask your pharmacist or doctor. The usual doses are described below.
Adults
Essential hypertension and renal parenchymal hypertension: 20-40 mg orally once daily. A dose of up to 80 mg/day has been reported to be safe and effective in clinical trials.
Angina: 40 mg/day.
Children
EFNOCAR is not recommended in infants and children as safety is not established in this group of patients.
Elderly
EFNOCAR should be started with a low dose (20mg/day). Your doctor will closely monitor your response to any decrease in the dose.
Patients with liver disease
Your doctor may give you a different dose to normal.
If you take more EFNOCAR Tablets than you should
If you (or someone else) swallow a lot of tablets all together, or if you think a child has swallowed any of the tablets, contact your nearest hospital casualty department or your doctor immediately. Take your medication and the packaging with you to the doctor or casualty department. If you have taken an overdose, you may you may appear flushed (your skin will look red), or you may feel dizzy or faint. If blood pressure drop is severe enough shock can occur.
If you forget to take EFNOCAR Tablets
If you forget to take a tablet, take one as soon as you remember, unless it is nearly time to take the next one. Never take two doses together. Take the remaining doses at the correct time.
If you stop taking EFNOCAR Tablets
Take this medicine for as long as your doctor tells you to, as you may become unwell if you stop.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The common side effects are hot flushes, palpitations, facial flushing and headache. In addition, elevation in serum total cholesterol, ALT (SGPT), AST (SGOT) and BUN may occur. Other known side effects are as follows. Tell your doctor if you notice or are worried by any of the side effects listed.
Frequency 0.1 to < 5%
Increased AST (SGOT), ALT (SGPT), LDH, Alkaline Phosphatase
BUN rise, serum creatinine rise, proteinuria
Decreased hemoglobin, decreased hematocrit, decreased red blood cells
Rash, itching
Palpitations, chest pain, decreased blood pressure
Flushing of the face
Headache, sluggishness and light-headedness
Nausea, stomach discomfort, abdominal pain
Malaise, serum total cholesterol rise, CK (CPK) increased, uric acid increased, hypokalemia
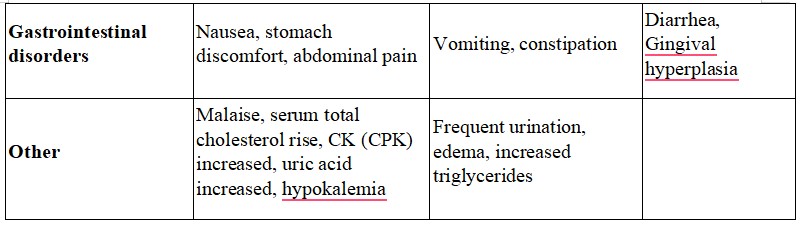
Frequency Less than 0.1%
- Increase in bilirubin
- Eosinophilia, leukopenia, thrombocytopenia
- Bradycardia, tachycardia, atrial fibrillation, premature ventricular contraction
- Hot flushes, sweating
- Drowsiness, numbness, and tinnitus
- Vomiting, constipation
- Frequent urination, edema, increased triglycerides
Frequency unknown
- Sick sinus syndrome, atrioventricular junctional rhythm, atrioventricular block, shock
- Diarrhea, Gingival hyperplasia
Tell your doctor or pharmacist if you notice any other effects not listed.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to Store Efnocar Tablets
Do not use the tablets after the end of the expiry month (use-by date) shown on the product packaging.
Store below 25°C. Protected from light & moisture.
Keep This Medicine Out of the Sight and Reach of Children
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the Pack and Other Information
What EFNOCAR Tablets contain
- The active substance is Efonidipine Hydrochloride Ethanolate.
Each tablet contains 10 mg / 20 mg / 40 mg of Efonidipine.
- Other ingredients: Excipients q.s.
Marketing authorisation holder:
Zuventus Healthcare Ltd.
Manufacturer responsible for batch release:
Zuventus Healthcare Ltd.
Kamerey Bhasmay, Elaka Pakyong,
Rangpo, East-Sikkim 737 132.
For More Information About This Product
Efnocar 20 Tablet
1.0 Generic Name
Efonidipine Hydrochloride Ethanolate Tablets 10 mg / 20 mg / 40 mg
2.0 Qualitative and quantitative composition
EFNOCAR-10
Each film coated tablet contains:
Efonidipine Hydrochloride Ethanolate 10 mg
Excipients q.s.
Colour: Titanium Dioxide IP
EFNOCAR-20
Each film coated tablet contains:
Efonidipine Hydrochloride Ethanolate 20 mg
Excipients q.s.
Colour: Titanium Dioxide IP
EFNOCAR-40
Each film coated tablet contains:
Efonidipine Hydrochloride Ethanolate 40 mg
Excipients q.s.
Colour: Titanium Dioxide IP
3.0 Dosage form and strength
Film coated tablet
10/ 20/40 mg
4.0 Clinical particulars
4.1 Therapeutic Indication
Efonidipine is indicated for the management of
- Essential hypertension and renal parenchymal hypertension
- Angina
4.2 Posology and method of administration
Adults
- Essential hypertension and renal parenchymal hypertension: 20-40 mg orally once daily. A dose of up to 80 mg/day has been reported to be safe and effective in clinical trials.
- Angina: 40 mg/day.
Elderly
Efonidipine should be started with a low dose (20mg/day) and the patient's condition should be monitored. The dose should be halved in case the patient is intolerant to the higher dose.
In Children
Efonidipine is not recommended in infants and children as safety is not established in this group of patients.
4.3 Contraindications
- In patients with known hypersensitivity to Efonidipine or any other component of the formulation
- In pregnant women
4.4 Special warnings and precautions for use
- Should be administered with caution in patients with hepatic impairment.
- Should be administered with caution in patients with low BP and/or sinus node dysfunction.
- The drug should be withdrawn gradually to prevent rebound hypertension or worsening of angina.
- Administration of the drug may cause hypotension. Under such circumstances appropriate measures should be taken to either reduce the dose or withdraw the drug.
- Should not be taken with grapefruit juice as there may be excessive lowering of blood pressure.
- Dizziness may occur while taking antihypertensive agents. Hence, working on aerial platform, working with dangerous machinery and/or driving should be avoided.
- To be sold by retail on the prescription of Cardiologist / Nephrologist / Endocrinologist and Specialist in General / Internal medicine / Critical Care medicine only.
4.5 Drugs interactions
Concomitant administration of other antihypertensive agent/s may enhance the antihypertensive effect of efonidipine.
- Administration of calcium channel blockers (CCBs) with Cimetidine may cause elevated levels of CCBs.
- Increased levels of CCBs observed when taken concomitantly with grapefruit juice which may result in excessive lowering of blood pressure.
- Efonidipine when taken along with Tacrolimus may cause increased blood levels of Tacrolimus.
4.6 Use in special populations
Pregnancy
Efonidipine should not be administered in pregnant women.
Nursing Mothers
Efonidipine should not be administered in lactating women.
4.7 Effects on ability to drive and use machines
If patients taking Efonidipine suffer from dizziness, headache, fatigue or nausea the ability to react may be impaired. Caution is recommended especially at the start of treatment.
4.8 Undesirable effects
The common side effects are hot flushes, palpitations, facial flushing and headache. In addition, elevation in serum total cholesterol, ALT (SGPT), AST (SGOT) and BUN may occur.
Tabulated list of adverse reactions


Reporting of suspected adverse reactions
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via email to:medico@zuventus.com
Website: https://www.zuventus.com/drug-safety-reporting
4.9 Overdose
In humans, experience with intentional overdose is limited. The stomach should be emptied by aspiration and gastric lavage if the patient reports immediately or up to two hours after overdosage.
Clinically significant hypotension due to Efonidipine overdosage calls for active cardiovascular support including frequent monitoring of cardiac and respiratory function, elevation of extremities, and attention to circulating fluid volume and urine output. A vasoconstrictor may be helpful in restoring vascular tone and blood pressure, provided that there is no contraindication to its use. Intravenous calcium gluconate may be beneficial in reversing the effects of calcium channel blockade.
5.0 Pharmacological properties
5.1 Mechanism of Action
Efonidipine, a new generation dihydropyridine (DHP) calcium channel blocker, inhibits both L-type and T-type calcium channels.
5.2 Pharmacodynamic properties
- Efonidipine exhibits antihypertensive effect through vasodilatation by blocking L-type and T-type calcium channels.
- Efonidipine has a negative chronotropic effect. Working on sino atrial node cells by inhibiting T-type calcium channel activation, Efonidipine prolongs the late phase-4 depolarization of the sino atrial node action potential and suppresses an elevated HR. The negative chronotropic effect of Efonidipine decreases heart rate, myocardial oxygen demand and increases coronary blood flow.
- Efonidipine increases coronary blood flow by blocking L & T-type calcium channels and attenuates myocardial ischaemia.
- By reducing synthesis and secretion of aldosterone, Efonidipine prevents hypertrophy and remodeling of cardiac myocytes.
- Efonidipine increases glomerular filtration rate without increasing intra-glomerular pressure and filtration fraction. This prevents hypertension induced renal damage.
- Efonidipine prevents Rho-kinase and NF-kB induced renal parenchymal fibrosis and provides long term renal protection.
- Efonidipine suppresses renin secretion from the Juxta Glomerular apparatus in the kidneys. •Efonidipine enhances sodium excretion from the kidneys by suppressing aldosterone synthesis and secretion from the adrenal glands. Aldosterone induced renal parenchymal fibrosis is suppressed by Efonidipine. •Efonidipine prevents NF-kB induced hypertrophy and inflammation in the renal vasculature and protects the kidneys.
- Efonidipine protects against endothelial dysfunction due to its anti-oxidant activity and by restoring nitric oxide bioavailability.
- Efonidipine has anti-atherogenic activity and protects the blood vessels from atherosclerosis.
- Efonidipine lowers blood pressure in cerebral resistance vessels and prevents hypertension induced brain damage.
5.3 Pharmacokinetic properties
Absorption
Peak plasma concentration is achieved in about 1.5 to 3.67 hours after administration. The bioavailability of Efonidipine is ~25% and half-life is approximately 4 hours.
Efonidipine 40mg when administered to healthy volunteers under fasting conditions shows the following pharmacokinetic profile:

The dissociation constant of Efonidipine from dihydropyridine receptors is very low (0.0042/min/nM), signifying very slow dissociation from the receptors. This explains the long duration of action of Efonidipine.
Metabolism
Efonidipine is primarily metabolized in the liver. The important metabolites are N-dephenylated Efonidipine (DPH), deaminated Efonidipine (AL) and N-debenzylated Efonidipine (DBZ). DBZ and DPH exhibit activity as calcium antagonists. The vasodilating properties of DBZ and DPH were about two-thirds and one-third respectively than that of the parent compound. Results suggest that the majority of the pharmacological effect after oral dosing of Efonidipine hydrochloride in man is due to unchanged compound and its metabolites make a small contribution to the pharmacological effect.
Elimination
Biliary route is the main pathway of excretion. No significant amount of unchanged drug is excreted in urine. In the urine collected for 24 h after an oral dosing, 1.1% of the dose was excreted as deaminated Efonidipine, and 0.5% as a pyridine analogue of deaminated Efonidipine.
6.0 Nonclinical properties
6.1 Animal Toxicology or Pharmacology
The acute toxicology of efonidipine was studied in male and female mice, rats, and dogs following oral or intravenous administration. The intravenous LD, values in male mice and rats were 77 and 51 mg/kg, respectively. The oral LD, values for efonidipine in mice, rats, and dogs were higher than 1,500 mag. The signs of toxicity observed in mice and rats were depression of spontaneous movement, hypothermia, sedation, piloerection, lying, gasping, and clonic convulsion. The dogs developed mild diarrhea, soft stools, or a temporary decrease in food consumption.
A 13-week oral subacute toxicity study of efonidipine was performed in male or female rats and dogs. Increases in water consumption and urine volume were observed in male and female rats receiving efonidipine at 300 mg/kg/day. Urinalysis revealed a high concentration of Na+ and C1- in the rats receiving 100 or 300 mg/kg/day. Organ weight measurement revealed an increase in liver and heart weight in male animals receiving 10 mg/kg/day or more and in female animals receiving 30 mg/kg/day or more.
Thus, in this study, the noneffective dose level was 3 mg/kg/day in rats. The effects observed at 300 or 100 mg/kg/day in groups of male or female dogs included reversible hyperemia of the sclera. Pathological examination revealed a dose-induced increase in the absolute and relative heart weight in female dogs treated with the drug at 100 mg/kg/day or more. Thus, the maximum noneffective dose of efonidipine in dogs of either sex was estimated to be around 30 mg/kg/day.
An oral chronic toxicity study of efonidipine was carried out in rats and dogs. Efonidipine was administered to rats at a daily dose of 1.5, 7, or 30 mg/kg for one year.
Organ weight measurement revealed an increase or a tendency of increase in heart weight in male or female rats receiving 30 mg/kg/day. A decreased thyroid weight in the males and an elevated liver weight in the females were found in the 30 mg/kg/day group.
Therefore, the noneffective estimated oral dose of efonidipine in rats was 7 mg/kg/day. Efonidipine in a gelatin capsule containing 2.0, 6.5, or 20 mg/kg/day was administered to dogs once daily for one year. The estimated effective dose was ca 20 mg/kg/day and the noneffective dose was ca 6.5 mg/kg/day
7.0 Description
Efonidipine is a novel dihydropyridine calcium channel blocker having anti-hypertensive and anti-anginal properties.
Chemical name: (±) -2 - [benzyl (phenyl) amino] ethyl 1, 4-dihydro-2 ,6-dimethyl- 5-(5,5-dimethyl-2-oxo-1 ,3,2-dioxaphosphorinan-2-yl) -4 - (3-nitrophenyl) -3-pyridinecarboxylate hydrochloride ethanol.
Molecular formula: C34H38 N3O7P • HCl •C2H6O
Molecular mass: 714.18 g/mol

8.0 Pharmaceutical particulars
8.1 Incompatibilities
Not applicable.
8.2 Shelf-life
Refer on pack
8.3 Packaging information
10 Blister strips of 10 tablets each
8.4 Storage and handing instructions
- Store below 25°C. Protected from light & moisture.
- Keep out of reach of children.
9.0 Patient Counselling Information
Efnocar is a type of medicine known as a calcium channel blocker (CCB). It is used to treat high blood pressure (hypertension) and a type of chest pain called angina. It can be used by itself or with other medicines to treat these conditions. Do not use Efnocar if you are allergic to efonidipine (the active ingredient in Efnocar), or to the inactive ingredients.
Tell your doctor about any prescription and non-prescription medicines you are taking, including natural or herbal remedies. Tell your doctor if you:
- ever had heart disease
- ever had liver problems
- are pregnant, or plan to become pregnant.
- are breast-feeding. Do not breast-feed while taking Efnocar.
You can stop breast-feeding or take a different medicine.
It may be easier to take your dose if you do it at the same time every day, such as with breakfast or dinner, or at bedtime.
While you are taking Efnocar do not stop taking your other prescription medicines, including any other blood pressure medicines, without talking to your doctor.
12.0 Date of revision
09/10/2024
About Leaflet
The name of your medicine is EFNOCAR 10 mg / 20 mg / 40 mg Tablets. We refer to them as EFNOCAR Tablets or EFNOCAR throughout this leaflet
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any more questions, please ask your doctor or your pharmacist.
This medicine has been prescribed for you personally and you should not pass it on to anyone else. It may harm them, even if their symptoms are the same as yours.
If any of the side effects get serious, or if you notice any side effects that are not listed in the leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What EFNOCAR Tablets are and what they are used for
2. What you need to know before you take EFNOCAR Tablets
3. How to take EFNOCAR Tablets
4. Possible side effects
5. How to store EFNOCAR Tablets
6. Contents of the pack and other information
1. What Efnocar Tablets Are and What They Are Used for
EFNOCAR Tablets contain the active substance efonidipine which belongs to a group of medicines called calcium antagonists.
EFNOCAR Tablets may be used to treat:
- High blood pressure (hypertension)
- hypertension caused by kidney disease
- A certain type of chest pain called angina
In patients with high blood pressure, these medicines work by relaxing blood vessels, so that blood passes through them more easily. In patients with angina, EFNOCAR works by improving blood supply to the heart muscle which then receives more oxygen and as a result chest pain is prevented. EFNOCAR Tablets do not immediately relieve chest pain caused by angina.
2. What You Need to Know Before You Take Efnocar Tablets
Do not take EFNOCAR Tablets if you:
- Have ever had an allergic reaction to efonidipine or any of the ingredients in the tablet. An allergic reaction may include a rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue;
- If you are pregnant
Take special care with EFNOCAR Tablets
You should inform you doctor if you have or have had any of the following conditions:
Liver disease;
Recent heart attack;
Heart failure;
Severe increase in blood pressure (Hypertensive crisis).
Use in children and adolescents
EFNOCAR is not recommended in infants and children as safety is not established in this group of patients.
For more information, talk to your doctor.
Taking other medicines and EFNOCAR
Please tell your doctor or pharmacist if you are taking or have recently taken other medicines, including medicines obtained without a prescription.
EFNOCAR may affect or be affected by other medicines, such as:
- Other antihypertensive agent/s (BP lowering drugs)
- Cimetidine (stomach acid reducer)
- Tacrolimus (a drug to suppress immunity)
EFNOCAR may lower your blood pressure even more if you are already taking other medicines to treat your high blood pressure.
If you see another doctor or go into hospital for any reason, tell them that you are taking EFNOCAR Tablets.
Taking EFNOCAR Tablets with food and drink
You should not drink grapefruit juice or eat grapefruit while taking this medicine. Grapefruit and grapefruit juice can lead to an increase in the blood levels of efonidipine, which can cause an unpredictable increase in its blood pressure lowering effect.
Pregnancy
The safety of efonidipine in human pregnancy has not been established. Efonidipine should not be administered in pregnant women.
Breast-feeding
It is not known whether efonidipine is passed into breast milk. Efonidipine should not be administered in breast-feeding women.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Dizziness may occur while taking antihypertensive agents. Hence, working on aerial platform, working with dangerous machinery and/or driving should be avoided.
3. How to Take Efnocar Tablets
Swallow these tablets with a glass of water at the same time each day. You can take the tablets after meals.
Follow your doctor’s instructions. Check the pharmacy label to see how many tablets to take and how often to take them. If you are still not sure, ask your pharmacist or doctor. The usual doses are described below.
Adults
Essential hypertension and renal parenchymal hypertension: 20-40 mg orally once daily. A dose of up to 80 mg/day has been reported to be safe and effective in clinical trials.
Angina: 40 mg/day.
Children
EFNOCAR is not recommended in infants and children as safety is not established in this group of patients.
Elderly
EFNOCAR should be started with a low dose (20mg/day). Your doctor will closely monitor your response to any decrease in the dose.
Patients with liver disease
Your doctor may give you a different dose to normal.
If you take more EFNOCAR Tablets than you should
If you (or someone else) swallow a lot of tablets all together, or if you think a child has swallowed any of the tablets, contact your nearest hospital casualty department or your doctor immediately. Take your medication and the packaging with you to the doctor or casualty department. If you have taken an overdose, you may you may appear flushed (your skin will look red), or you may feel dizzy or faint. If blood pressure drop is severe enough shock can occur.
If you forget to take EFNOCAR Tablets
If you forget to take a tablet, take one as soon as you remember, unless it is nearly time to take the next one. Never take two doses together. Take the remaining doses at the correct time.
If you stop taking EFNOCAR Tablets
Take this medicine for as long as your doctor tells you to, as you may become unwell if you stop.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The common side effects are hot flushes, palpitations, facial flushing and headache. In addition, elevation in serum total cholesterol, ALT (SGPT), AST (SGOT) and BUN may occur. Other known side effects are as follows. Tell your doctor if you notice or are worried by any of the side effects listed.
Frequency 0.1 to < 5%
Increased AST (SGOT), ALT (SGPT), LDH, Alkaline Phosphatase
BUN rise, serum creatinine rise, proteinuria
Decreased hemoglobin, decreased hematocrit, decreased red blood cells
Rash, itching
Palpitations, chest pain, decreased blood pressure
Flushing of the face
Headache, sluggishness and light-headedness
Nausea, stomach discomfort, abdominal pain
Malaise, serum total cholesterol rise, CK (CPK) increased, uric acid increased, hypokalemia
Frequency Less than 0.1%
- Increase in bilirubin
- Eosinophilia, leukopenia, thrombocytopenia
- Bradycardia, tachycardia, atrial fibrillation, premature ventricular contraction
- Hot flushes, sweating
- Drowsiness, numbness, and tinnitus
- Vomiting, constipation
- Frequent urination, edema, increased triglycerides
Frequency unknown
- Sick sinus syndrome, atrioventricular junctional rhythm, atrioventricular block, shock
- Diarrhea, Gingival hyperplasia
Tell your doctor or pharmacist if you notice any other effects not listed.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly: Website: www.zuventus.co.in and click the tab “Safety Reporting” located on the top right end of the home page.
By reporting side effects, you can help provide more information on the safety of this medicine.
You can also report the side effect with the help of your treating physician.
5. How to Store Efnocar Tablets
Do not use the tablets after the end of the expiry month (use-by date) shown on the product packaging.
Store below 25°C. Protected from light & moisture.
Keep This Medicine Out of the Sight and Reach of Children
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the Pack and Other Information
What EFNOCAR Tablets contain
- The active substance is Efonidipine Hydrochloride Ethanolate.
Each tablet contains 10 mg / 20 mg / 40 mg of Efonidipine.
- Other ingredients: Excipients q.s.
Marketing authorisation holder:
Zuventus Healthcare Ltd.
Manufacturer responsible for batch release:
Zuventus Healthcare Ltd.
Kamerey Bhasmay, Elaka Pakyong,
Rangpo, East-Sikkim 737 132.


